(To sign up for a free subscription to Food Safety News, please click here.)
Several weeks ago, food safety advocate Bill Marler launched his campaign to “Get the ‘F’ out of the FDA.”
Marler proposed that Congress split the Food and Drug Administration into two separate agencies — one with responsibility for food safety and human nutrition, and the other for drugs, cosmetics and medical devices.
While I agree with the need for a separate agency to oversee food safety and human nutrition, I believe Marler’s proposal does not go far enough.
It is time to consolidate all food safety activities at the federal level under a single umbrella.
Here’s why.
Split jurisdiction
At present, responsibility for overseeing food safety is split between two main federal agencies: the FDA and the Food Safety and Inspection Service (FSIS) of the U.S. Department of Agriculture (USDA).
The USDA is responsible for the safety of meat and poultry, catfish, and egg products.
The FDA is responsible for the safety of all food products that do not contain meat or poultry, for intact eggs, and for all fish and seafood other than catfish.
This division of responsibility by commodity has led to some strange and confusing situations.
Canned foods containing meat or poultry come under FSIS jurisdiction, while all other canned foods are FDA-regulated.
Pizzas containing more than 2 percent meat are the responsibility of FSIS; less than 2 percent meat, and the FDA takes over.
Open-faced sandwiches containing meat are overseed by FSIS; closed sandwiches are the responsibility of the FDA, whether or not meat is present.
These arbitrary distinctions mean that many food processing plants must answer to two separate federal agencies.
Conflict of interest
The USDA operates under a double mandate.
On the one hand, it is responsible for certifying that the food products under its jurisdiction are safe for human consumption.
On the other hand, the USDA also is charged with promoting U.S. agricultural products both domestically and to overseas markets.
This is akin to having the quality assurance department of a food company report to the head of the marketing department.
We have seen the consequences of this conflict most recently in the FSIS draft proposal to allow as much as one Salmonella per gram of chicken in raw, breaded stuffed chicken products.
An immodest proposal
It is time to demolish the current ineffective, wasteful, and conflicted system and build a new one, centered on a new Food Safety and Nutrition Agency (FSNA) with a seat at the Cabinet table.
The FSNA would take over all of the food safety and nutrition program activities currently performed by the FDA. In addition, all responsibility for meat, poultry, egg products and catfish would fall under the FSNA umbrella.
The USDA would retain responsiblity for certifying the fitness of livestock for slaughter and certifying the fitness of their meat for human consumption.
At the moment meat or poultry leaves the slaughterhouse, jurisdiction would shift to the FSNA.
This approach would have the benefit of eliminating the conflict of interest inherent in the USDA’s double mandate. It would also unscramble the arbitrary and confusing overlap of jurisdictions between the FDA and the FSIS.
The consolidation of all food safety responsibilities within a single, independent agency is not a new idea.
The Canadian Food Inspection Agency (CFIA) was created in 1997 by consolidating into a single agency the food safety components of the Health Protection Branch (then the Canadian equivalent to the FDA), the Department of Agriculture, and the Department of Fisheries and Oceans.
Other countries, including the United Kingdom, Australia, and New Zealand have followed a similar path.
The bottom line
Congress created the current dysfunctional structure over a span of many decades.
Therefore, it is up to Congress to deconstruct this broken system and build a new one that will work to the benefit of the public it has been elected to represent.
Editor’s note: This column was originally published in eFoodAlert and is reprinted here with permission.
(To sign up for a free subscription to Food Safety News,click here)
]]>CONTRIBUTED
Editor’s note: This was originally posted by eFoodAlert and is reposted here with the permission of the author. To view the document from the FDA, click here.
Between December 1, 2021, and March 3, 2022, the US Food and Drug Administration (FDA) received nine (9) reports of infant deaths among babies who were fed powdered infant formula manufactured by Abbott Nutrition in Sturgis, Michigan.
The infant death reports were included in a list of 128 consumer complaints supplied to eFoodAlert by the FDA in response to a Freedom of Information Act (FOIA) request. (see: Abbott Nutrition consumer complaints file from the FDA.)
Two of the deaths were numbered among the four confirmed outbreak cases of Cronobacter sakazakii identified by the US Centers for Disease Control and Prevention (CDC).
The other seven deaths were reported to the FDA via the agency’s consumer complaint system. Two of those reports mentioned Salmonella in the complaint description.
In addition to the nine deaths, consumers described twenty-five (25) incidents categorized as “Life Threatening Illness/Injury” and eighty (80) instances of “Non-Life Threatening Illness/Injury.”
Fourteen consumers contacted the FDA to obtain information or clarifications on the Abbott recall.
The complaints were lodged with FDA District Offices across the continental USA.
Salmonella was present in two of the dead babies, and was mentioned in seventeen other illness complaints.
One of the surviving infants was infected with both Salmonella and E. coli.
The symptoms suffered by the infants were mostly consistent with a gastrointestinal infection: fever (31 babies), vomiting (42 babies), diarrhea (47 babies), and blood in stool (6). Most babies suffered from multiple symptoms.
Other reported symptoms included loss of appetite, rash (either localized or spread over entire body), lethargy, dehydration, irritability, weight loss, and difficulty breathing.
Some of the infants suffered from multiple infections:
- Cronobacter sakazakii and Proteus mirabilis
- Covid-19 and Salmonella
- CDIFF (Clostridioides difficile) and Salmonella
- Salmonella and Shigella
- Salmonella, astrovirus, and “shigelloides”
The FDA did not respond to eFoodAlert‘s request for comment on what was done to follow up on the seven infant death reports that did not involve Cronobacter sakazakii, or on the non-fatal illnesses not involving Cronobacter.
According to an agency spokesperson, the FDA, along with the CDC and state and local partners, investigated consumer complaints and/or reports, received from September 20, 2021 to February 24, 2022, of infant illness.
The spokesperson did not address any of the more than thirty complaints–including three reported deaths–received by FDA district offices between February 25, 2022 and March 3, 2022.
Two of those three reported deaths referred to Salmonella.
The FDA investigation uncovered multiple instances of Cronobacter sakazakii in the environment of Abbott’s manufacturing facility.
None of the cultures retrieved from environmental samples were a genetic match for the strain that infected the two babies for which the CDC received cultures. The CDC did not receive cultures from the other two infected babies for genetic analysis.
Although there is no direct evidence in the form of genome sequencing to link any of the illnesses unequivocally to Abbott’s infant formulas, all of the complaints have one element in common.
Every one of the sick babies was fed an Abbott powdered formula.
The FDA has established an Incident Management Group (IMG) under Frank Yiannas, FDA Deputy Commissioner for Food Policy and Response.
The IMG is tasked with managing the ongoing investigation and monitoring the infant formula supply chain, and will remain in place at least until the current supply shortage is over, according to the FDA spokesperson.
With production now having resumed–under close supervision–at the Abbott Nutrition plant in Sturgis, the time has come to acknowledge the lives that were lost.
We know very little about the nine infants who died. For privacy reasons, their names, ages, genders, where they lived, and when they died have been withheld. They are identified only by their unique Complaint ID numbers.
In Memoriam
Complaint ID #171222, reported December 1, 2021. Infant arrived to the ER in cardiac arrest. Cronobacter sakazakii and Proteus mirabilis. Infant had consumed Similac Pro-Total Comfort (Powder) infant formula, Lot #23495K80.
Complaint ID #172435, reported February 22, 2022. Vomiting, swollen organs, trouble breathing. Infant had consumed Similac Advance, Lot #34875K80.
Complaint ID #172477, reported February 22, 2022. Screaming. Infant had consumed Similac Total Comfort Easy-to-Digest Gentle Protein & Prebiotics, et al, infant formula powder, Lot #34869K80.
Complaint ID #172479, reported February 23, 2022. Fever, diarrhea, loss of appetite, vomiting. Infant had consumed Similac Advanced infant formula. Lot number not available.
Complaint ID #172541, reported February 24, 2022. Tested positive for Cronobacter sakazakii. Infant had consumed Similac PM 60/40, Lot #27032K800.
Complaint ID #172585, reported February 24, 2022. No details available. Infant had consumed EleCare infant formula, Lot number not available.
Complaint ID #172607, reported February 28, 2022. Cause of death and opinion pending further studies (Congenital). Infant had consumed Similac Elecare powdered infant formula, Lot #34771Z21 1306305
Complaint ID #172632, reported March 2, 2022. Salmonella meningitis, ventriculitis, vomit, diarrhea, seizures, bradycardia. Infant had consumed Similac Pro Advance infant formula, Lot #25598SHO 0557 015 SIMESPWD.
Complaint ID #172636, reported March 2, 2022. Salmonella. Infant had consumed Similac Total Comfort, Lot #26834K80.
May they rest in peace.
]]>Editor’s note: This column was originally published in eFoodAlert and is republished here with the author’s permission.
Between Sept. 1, 2019, and Sept. 20, 2021, Abbott Nutrition received 17 consumer complaints regarding multiple Similac powdered infant formula products.
Fifteen of the complaints related to infants testing positive for Salmonella after consuming a Similac product. One complaint cited an infant who was diagnozed with Cronobacter (Enterobacter) sakazakii, and one was as the result of an infant death from an unspecified cause.
This information is contained in the Sept. 20-24, 2021, Establishment Inspection Report (EIR), obtained by eFoodAlert from the Food and Drug Administration in response to a Freedom of Information Act request.
How the complaints were handled
In response to the Cronobacter complaint, the company reviewed its batch records and its finished product microbiological testing records. The complaint was reviewed by an internal Abbott Nutrition Medical Team.
The firm closed the complaint after determining that all batch records were acceptable, that there were no other consumer complaints, and that microbiological testing was negative for C. sakazakii.
The infant death complaint triggered a batch record review for three lots of Similac Alimentum. After completing the review and determining that no other complaints or medical concerns had been identified for the products, the company closed the complaint.
The 15 Salmonella complaints involved infants who had been fed one or more batches of Similac Alimentum, Similac ProAdvance, Similac Spit-Up, Similac Total Comfort, Similac Advance, Similac Pro Sensitive or Elecare for Infants.
All 15 of the infants tested positive for Salmonella.
Once again, the batch record reviews came back acceptable, and finished product testing results were negative for Salmonella.
A finished product sample of one of the implicated batches (Similac Advance lot #472005) was obtained by Abbott Nutrition from the consumer. The sample was subjected to a visual exam, and the container was examined in the packaging lab.
The company did not conduct any microbiological tests on this sample.
Abbott Nutrition’s “Standard Operating Procedure for Handling Complaints” specifies that “. . . any chemical or microbial testing of an unopened customer sample requires the approval of the AN Vice President Quality or delegate.”
The EIR does not state whether approval was sought to carry out microbiological tests on the sample.
Abbott’s internal test results raise concerns
In addition to the consumer complaints, the EIR also reveals that the company had found Cronobacter in two batches of finished product.
The first of these positive results was recorded for Similac Alimentum (Batch 697464), produced on Sept. 25, 2019, just one day after the completion of the FDA’s September 2019 inspection of Abbott’s production facility.
The root cause of the contamination was determined to be environmental. The company implemented correction actions and destroyed the contaminated batch.
The specific root cause for the second positive result, this time in Similac Spit-Up (Batch 732675), produced on June 22, 2020, was never found, according to the EIR. Several deficiencies were noted during the root cause investigation, corrective actions were implemented, and the batch was destroyed.
In addition to the two instances of Cronobacter in finished products, Abbott also found Cronobacter in five environmental samples between January 2019 and August 2021. There were no Salmonella-positive environmental samples.
All of the Cronobacter-positive results were from non-product contact surfaces.
In its Feb.17, 2022, recall notice, Abbott acknowledged “…evidence of Cronobacter sakazakii in the plant in non-product contact areas.”
Yet the FDA investigation recovered Cronobacter sakazakii from at least one swab of what appears to be a contact surface, as described in the 1/31/2022-3/18/2022 Inspectional Observations report (FDA Form 483).
The explanation for the absence of Cronobacter-positive findings on product contact surfaces is revealed in the description of Abbott Nutrition’s environmental sampling procedures as reported in the September 2021 EIR.
According to the description of Abbott Nutrition’s environmental sampling program, the company conducts environmental sampling of product contact surfaces and non-product contact surfaces, as well as air, water, steam and compressed air.
Swab samples from product contact surfaces and non-product contact surfaces are tested for Enterobacteriaceae.
Enterobacteriaceae is a family of bacteria that includes both Salmonella and Cronobacter, and a test for total Enterobacteriaceae may be used as an indicator of general sanitary conditions in a production facility.
If a non-product contact surface produced a positive result in an area of the plant that was considered “high care” by the company, the isolates were analyzed for both Salmonella and Cronobacter.
On the other hand, if a product contact surface was positive for Enterobacteriaceae, the company did NOT test the isolates for Salmonella or Cronobacter, rationalizing that the finished product is analyzed for both microbes.
However, except in the event of massive contamination, Salmonella or Cronobacter most likely would be present at very low levels in the finished product, and the chances of detecting these contaminants would be akin to having the same number come up twice in a row on a roulette wheel.
By choosing not to test Enterobacteriaceae-positive product contact surfaces for Cronobacter or Salmonella, the company missed an opportunity to head off a serious problem.
FDA not blameless
There was a two year gap between inspections of Abbott Nutrition’s production plant in Sturgis, Michigan.
During this time, the United States — indeed, the entire world — was reeling from the Covid-19 pandemic.
When the FDA returned to Abbott, the company’s Covid-19 protection program required that the agency give advance notice of their planned inspection — something that had not been the case in the past.
Although the company had four days notice during which they could “tidy up” in anticipation of the FDA visit, the inspection team still found several issues of note, which were detailed in the Inspectional Observations form (FDA Form 483) provided to the company at the end of the inspection.
But one key observation was missing from the list:
There was no mention of the two finished product batches that had tested positive for Cronobacter sakazakii since the previous inspection, nor of the Cronobacter-positive environmental test results.
These observations were included instead on the Form 483 issued at the end of the January-March 2022 inspection.
According to the September 2021 EIR, the two-person FDA inspection team did not carry out any environmental sampling during the course of their visit, even after learning of the Cronobacter-positive results. Two finished product batches were sampled for nutrient analysis and two for microbiological analysis.
Unanswered questions
Seven months after FDA received the first report of an infant infected with Cronobacter and nearly four months after the agency initiated its in-depth inspection of Abbott Nutrition’s production facility, several questions remain:
- In view of what the FDA learned in September 2021 regarding Cronobacter-positive environmental and finished product samples at the Abbott facility, why did it take more than four months for the agency to initiate another inspection after receiving the first of the illness reports?
- Why did the FDA inspectors not respond more forcefully to those Cronobacter-positive results when writing up the list of Inspectional Observations at the completion of their September 2021 inspection?
- Would Abbott have discovered and addressed its contamination problem sooner if it had tested product-contact surfaces for Cronobacter instead of relying upon finished product tests?
- As Cronobacter (unlike Salmonella) is not a “reportable” disease in 49 states, how many additional cases of Cronobacter in infants have gone unreported?
- Why did it take until Feb. 17, 2022, before the public was made aware of the situation?
(To sign up for a free subscription to Food Safety News, click here)
]]>The warning letter to JBS Souderton Inc. which does business as MOPAC was sent more than one year after pentobarbital was first discovered in beef tallow from the company’s Souderton, PA, facility.
Pentobarbital is a barbiturate used by veterinarians to euthanize animals, including companion animals, horses and cattle. According to the FDA, pet foods containing even a trace amount of pentobarbital are considered adulterated. It is against federal law to release “adulterated” products into the stream of commerce.
JBS was the supplier of beef tallow to Big Heart Pet Brands Inc. and to Champion PetFoods, among others.
Big Heart is a wholly owned subsidiary of The J.M. Smucker Company Inc. Champion is a Canadian pet food company whose U.S. production facility is in Auburn, KY. It manufactures Acana and Orijen brands of dry dog food.
In February 2018, a media outlet reported having found pentobarbital in several samples of Gravy Train canned, wet dog food. Smucker initiated a product withdrawal of the implicated products pending the outcome of its internal investigation.
Concurrently, FDA alerted pet owners about the possible presence of pentobarbital in the several dog food brands, including Gravy Train, Kibbles ’N Bits, Ol’ Roy and Skippy.
Smucker converted its withdrawal into a full-blown recall once company officials had confirmation of the presence of pentobarbital in its finished product and in samples of beef tallow supplied by JBS.
As part of its investigation into the Big Heart, FDA and the Pennsylvania Department of Agriculture conducted a joint inspection of JBS beginning March 13, 2018.
According to the warning letter, FDA found pentobarbital in four out of nine samples collected at the JBS facility. Upon further analysis, three of the samples were found to contain pentobarbital at levels ranging from 61.8 +/-19 to 277 +/-70 nanograms per gram (ng/g), well above the minimum detection concentration of 4 ng/g.
The four pentobarbital-contaminated products were delivered to customers from November 2017 through March 2018.
Samples collected from JBS and from its customers’ facilities and analyzed by Pennsylvania officials found levels of pentobarbital as high as 680 ng/g.
The list of Inspectional “Observations” in the FDA’s Form 483, provided to JBS management on Oct. 17, 2018, contained two items:
- JBS did not visually verify loads of raw materials with what the hauler stated that they brought in. This led to tallow, manufactured at [the JBS] facility, to be adulterated with pentobarbital.
- JBS did not have an effective system for evaluating incoming raw materials to ensure that these ingredients are suitable for use in human products and animal feeds.
JBS officials informed the FDA on April 17, 2018, that the company had completed cleaning all of its conveyances, conduits, cookers and centrifuges, and some of its storage tanks to remove any pentobarbital-contaminated product. In a May 30, 2018, letter, JBS management indicated the company would complete the cleaning process within an additional 30 days.
The company officials also reported having identified and talked with all of its suppliers that may have presented a risk for entry of euthanized animals into the rendering plant, and obtained a guarantee from each supplier that they would not provide euthanized animals. JBS also indicated it would continue to conduct random tests of tallow products for pentobarbital.
On July 27, 2018, the FDA took a follow-up sample from one of the JBS storage tanks. Upon analysis, the sample was found to contain trace amounts of pentobarbital.
On Aug. 8, 2018, the FDA inquired what actions JBS planned to take in response to the pentobarbital finding.
The company declined to recall the product. Instead, JBS offered to ask animal food producing customers that received animal food products to remove any products deemed positive for pentobarbital and to have their tanks cleaned.
JBS described its product withdrawals and attempted withdrawals of pentobarbital-contaminated product from its customers in a Nov. 26, 2018, letter to the FDA.
In its warning letter, the FDA noted that it was unable to asses the effectiveness of the corrective actions in the absence of a voluntary recall or other documentation demonstrating all contaminated products were removed from the marketplace.
As reported by Food Safety News in November 2018, Champion PetFoods retrieved pet foods the contaminated tallow from its third-party distributors. The company declined to initiate a retail-level recall, even though some of the product had reached the store/consumer level. The refusal was based on laboratory test results on retained samples of those finished products that did not reveal pentobarbital.
JBS was given fifteen working days to notify the FDA in writing of the specific steps it has taken to correct the violations listed in the warning letter, or to provide a time frame within which the corrections will be completed.
(To sign up for a free subscription to Food Safety News, click here.)
]]>Most of the outbreak victims reported eating pre-cut cantaloupe, watermelon or a fruit salad mix with melon purchased from grocery stores.
According to the CDC, epidemiologic and traceback evidence pointed to consumption of pre-cut melon supplied by Caito Foods LLC of Indianapolis.
In response to the evidence, the U.S. Food and Drug Administration (FDA) performed a three-week long investigation, including a comprehensive inspection of Caito’s production facility and analysis of several environmental and cut fruit samples.
FDA lab analysis did not reveal Salmonella in any of the samples taken during the course of the investigation. However, the Michigan Department of Agriculture and Rural Development (MDARD) found Salmonella Adelaide after testing samples of cut cantaloupe and watermelon.
Although FDA did not confirm the presence of Salmonella in Caito’s production facility during the inspection, investigators noted several sanitation and maintenance issues, according to the Establishment Inspection Report, obtained by Food Safety News as part of a Freedom of Information Act request.
- The plant was not designed to facilitate maintenance and sanitary operations. There was condensation from the cooling units and on electric cords located directly above the pineapple line, where fresh, ready-to-eat pineapples were being peeled and cut.
- The firm did not conduct operations under conditions and controls necessary to minimize the potential for contamination of food. Five employees were observed neglecting appropriate personal sanitizing procedures when entering the production area.
- The firm did not take a reasonable measure and precaution related to personnel practices. Employees were seen handling containers and packing materials and then returning to cut watermelon without first cleaning and sanitizing their hands.
- The cooling units’ fans appeared to be dirty.
- The firm used a lower concentration of sanitizing chemical than called for on the package label. Management explained that the sanitizer was used to ensure the safety of the water, and not to provide a sanitizing step for the fruit.
During the current inspection the firm on June 14, 2018, Caito Foods LLC voluntary destroyed all the melons and watermelons that they had in their warehouse and diverted any shipments that they had coming. The firm also destroyed any products containing melon or watermelon.
FDA never found the source of the Salmonella Adelaide contamination.
In its official response to FDA’s observations, Caito management detailed corrective actions intended to eliminate the condensation problem and prevent a recurrence. The company developed a new procedure regarding management, sanitation, and handling of all food contact containers, and completed a retraining program for employees on handling procedures and hand sanitizing requirements.
Caito also adjusted the concentration of sanitizing chemical.
Despite its corrective actions, Caito is once again the apparent source of a Salmonella outbreak. As of April 24, CDC had received confirmed reports of 117 cases of Salmonella Carrau infections in 10 states. Thirty-two people have been admitted to hospitals.
Epidemiologic and traceback evidence has identified Caito as the likely source of the outbreak.
According to FDA, Salmonella Carrau is rare, and has historically been seen in imported melons. Caito has acknowledged that imported melons were used in the suspect pre-cut melon mixes.
FDA is examining shipping records to establish a country of origin and, if possible, a farm of origin for the melons.
Caito recalled all of the implicated pre-cut melons and fruit mixes containing pre-cut melons on April 12.
Public health officials from CDC and FDA advise consumers to check their homes for recalled products and either throw them away or return them to the place of purchase. Consumers who believe they have become ill as a result of consuming pre-cut melon should consult their healthcare provider.
The outbreak, which was first announced on April 5, 2019, has spread to six Canadian provinces: British Columbia (27), Alberta (12), Saskatchewan (9), Manitoba (10), Ontario (13) and Quebec (2).
The outbreak began in early November 2018 and remains ongoing, with the most recent case having been reported in late March 2019. Outbreak victims range in age between 1 and 88 years of age.
PHAC has not determined whether or not Salmonella was a contributing factor in either of the deaths. Nineteen outbreak victims have been hospitalized.
Many of the victims reported eating Celebrate brand classic/classical or egg nog flavored profiteroles or mini chocolate eclairs purchased at various grocery stores before becoming ill.
The Canadian Food Inspection Agency (CFIA) has issued a Food Recall Warning for certain Celebrate brand products.
The implicated products were manufactured in Thailand by Mountain Mist (The Belgian Baker) Thailand Ltd. and distributed in Canada by Retail Resource Services Inc., located in Beaumont, Alberta, Canada (Retail Resource).
All lot codes of the following Celebrate brand products have been recalled so far. CFIA warns that more products may be recalled, depending on the outcome of its food safety investigation.
- Mini Chocolate Eclairs, 365g (UPC 8 858762 720047)
- Classical Profiteroles / Classic Profiteroles, 325g (UPC 8 858762 720009)
- Egg Nog Profiteroles, 375g (UPC 8 858762 720016)
- Classic Foodservice Profiteroles, 4 kg (No UPC)
- Pineapple Foodservice Profiteroles, 4 kg (No UPC)
- Coconut Foodservice Profiteroles, 4 kg (No UPC)
- Passionfruit Foodservice Profiteroles, 4 kg (No UPC)
- Mango Foodservice Profiteroles, 4 kg (No UPC)
The recalled products were sold in Alberta, British Columbia, Manitoba, New Brunswick, Nova Scotia, Ontario, Quebec, Saskatchewan and may have been distributed elsewhere in Canada.
Symptoms of Salmonella usually begin from 6 to 72 hours after exposure and may include the following: fever, chills, diarrhea, abdominal cramps, headache, nausea, and vomiting.
In healthy individuals, symptoms usually last from 4 to 7 days and often resolve without treatment. In some cases, more severe illness can occur. The very young, the elderly, pregnant women, and individuals with weakened immune systems are more susceptible to complications from Salmonella infections.
PHAC advises consumers to take the following precautions if they have purchased or been given one of the recalled products:
- Do not eat recalled Celebrate brand profiteroles (cream puffs) or mini chocolate eclairs.
- Throw them out immediately and properly wash and sanitize any containers that were used to store these products before using them again.
- If you have any profiteroles or mini eclair products without the original packaging and are unsure if these products are included in this advice, throw them out just to be safe.
- Wash your hands with soap and warm water for at least 20 seconds immediately following contact with any of the identified Celebrate brand products.
- Do not prepare food for other people if you think you are sick with a Salmonella infection or suffering from any other contagious illness causing diarrhea.
(To sign up for a free subscription to Food Safety News, please click here.)
According to the Indianapolis company’s website, Caito specializes in fresh produce distribution and fresh food processing, selling to to customers nationwide.
The September 2016 FDA inspection was undertaken following detection of Listeria monocytogenes in a sample of cut butternut squash by the Ohio Department of Agriculture (ODA), according to documents obtained from FDA in response to a Freedom of Information Act request.
The company decided not to recall the squash from the sampled lot, because the product was intended to be cooked by the consumer. Also, the company did not learn about the contamination until Sept. 9, 2016, which was five days after the “BEST IF SOLD BY” date for the batch.
FDA investigators spent two days on a “directed inspection” in response to the Listeria monocytogenes finding. Their inspection focussed on the processing of raw fruits and vegetables, including butternut squash.
The “Establishment Inspection Report” noted several observations, which were provided to management at the completion of the inspection.
- Condensate dripping onto uncovered asparagus spears on the over-wrap line during the, even though the pre-operation sanitation checklist indicated “No condensation” for that date.
- Pre-operation sanitation check list for the date during which the butternut squash sample was produced identified three locations as “unsatisfactory” with no corrective actions indicated.
- During the inspection, an employee on the production line was observed placing “. . . waste into a trash can under the product line, pushing the waste down into the can with their hand, until their arm from the elbow down was fully in the trash can.” The employee immediately returned to handling cut watermelon chunks without changing or sanitizing gloves.
- Condensate water formed a puddle on the floor at one of the entrances to the receiving cooler, a potential reservoir for Listeria, which could be tracked into the rest of the facility.
Caito’s production facility was inspected again by FDA in 2018, in response to an outbreak of Salmonella Adelaide infections linked to freshcut melon products. That outbreak sickened 77 individuals in nine states.
On April 12 this year, Caito recalled various pre-cut melons and fruit medley products after the products were linked to cases of salmonellosis. As of April 24, there were 117 confirmed patients in the 10-state outbreak. At least 32 of the patients have been admitted to hospitals, according to an update this week from the Centers for Disease Control and Prevention.
The FDA is conducting an on-going investigation to determine the cause of the outbreak, including a traceback investigation to determine, if possible, a farm of origin for the melons. According to an agency spokesperson, FDA’s inspection of Caito’s production plant is still in progress.
(To sign up for a free subscription to Food Safety News, click here.)
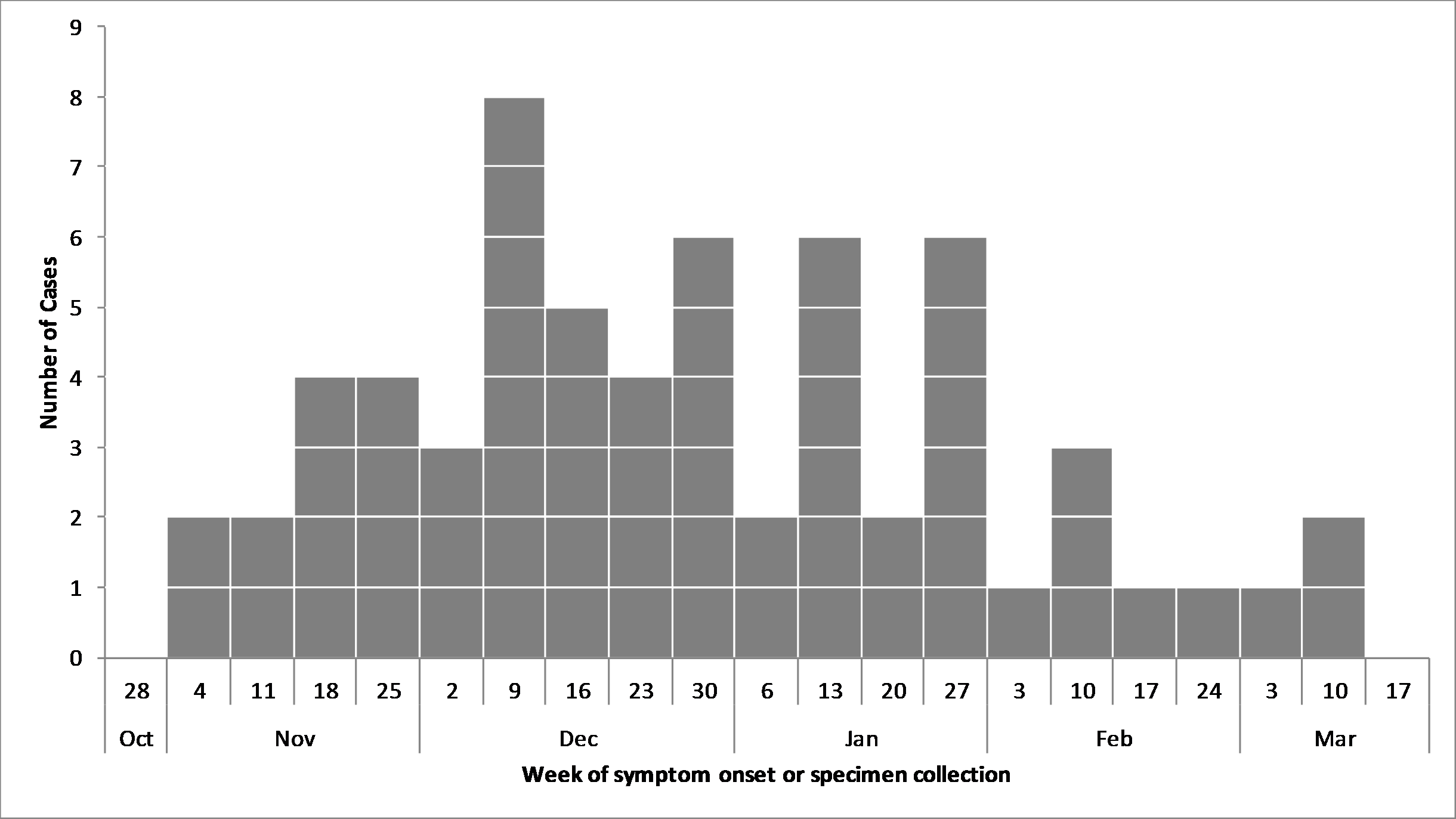
]]>PHAC, its provincial partners, and the Canadian Food Inspection Agency are collaborating in the investigation of this outbreak, which is apparently unrelated to two other outbreaks of Salmonella illnesses linked to raw chicken and raw turkey.
As of April 5, 2019, 63 laboratory-confirmed cases have been reported by provincial public health authorities in six provinces: British Columbia (23), Alberta (10), Saskatchewan (8), Manitoba (10), Ontario (10) and Quebec (2).
Figure 1: Number of people infected with Salmonella Enteritidis
The outbreak has affected individuals of all ages, from 1 year to 87 years of age; 57% of the victims are female.
The source of the outbreak has not yet been identified.
The first Salmonella illnesses related to this outbreak occurred in early November 2018, and new illnesses continue to be reported.
Symptoms of Salmonella infections typically develop six to 72 hours after exposure and may include fever, chills, diarrhea, cramps, headache, nausea and vomiting. The symptoms usually last from four to seven days; however, people who are infected with Salmonella may remain infectious from several days to several weeks after symptoms have disappeared.
Some individual can experience more severe illness that requires hospitalization.
Individuals who experience symptoms of a Salmonella infection should consult their health care provider.
(To sign up for a free subscription to Food Safety News, click here.)
The recall was triggered as a result of sampling by the Washington State Department of Agriculture.
Recalled products include two-pound packs of course ground rabbit, course ground mallard duck, ground llama, and ground pork frozen raw pet food.
The front of the packages bears a large, white square label with the company name, the product type, and product weight. No lot numbers, batch codes or expiration dates have been specified in this recall.
In addition to producing and selling raw dog food, Thogersen Family Farm breeds and shows rabbits, goats, dogs, ducks, and other fowl. The company sells its pet food to individual consumers and to two retail establishments.
Pets infected with Listeria monocytogenes may be lethargic and have diarrhea or bloody diarrhea, fever, and vomiting. Some pets will have only decreased appetite, fever, and abdominal pain. Infected pets may not show any symptoms at all, yet still, be carriers of the bacteria and infect other animals or humans.
Healthy individuals who become infected with Listeria monocytogenes may suffer only short-term symptoms such as high fever, severe headache, stiffness, nausea, abdominal pain, and diarrhea.
Listeria monocytogenes infections can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Listeria infection can cause miscarriages and stillbirths among pregnant women.
What consumers should do
• Do not feed any of the recalled products to your pet. Discard any recalled product in a secure location to keep it safe from children, pets, and wildlife
• If your pet has consumed the recalled product and has symptoms of Listeria monocytogenes infection, contact your veterinarian.
• If you or a member of your household is experiencing symptoms of Listeria monocytogenes infection, contact your health care provider.
• Customers with questions may contact the Thogersen Family Farm at (360) 929-9808.
(To sign up for a free subscription to Food Safety News, click here)
]]>
According to information obtained by Food Safety News in response to Freedom of Information Act (FOIA) requests, Hill’s identified Vitamin Premix as a ‘high risk’ chemical hazard and required that the ingredient “…be analyzed and be within acceptable limits prior to unloading … into the manufacturing facility.”
The company was unable to provide analytical test results for Vitamin Premixes during a February 2019 inspection.
The inspection was undertaken by the U.S. Food and Drug Administration (FDA) in response to the January 31, 2019 Hill’s recall of canned pet foods due to excess levels of vitamin D.
Tests conducted on a retained sample of the premix revealed a level of vitamin D that was roughly 30 times the target range for this ingredient. As of February 11, 2019, the company acknowledged having received 85 consumer complaints reporting pet deaths.
The number of complaints received by the company has increased substantially since that date, according to information supplied to FDA by Hill’s in response to FDA’s inspection observations.
FDA declined to comment on the number of pet deaths. According to an agency spokesperson, FDA is still in the process of verifying details of the complaints it has received, and considers it would be “…premature to release a number until the cases have been vetted to ensure they are all related to recalled product and are indeed cases of vitamin D toxicity.”
Hill’s cited a manufacturing error on the part of its vitamin premix supplier as the cause for the excessive vitamin D levels in its canned pet foods. The company is reevaluating and strengthening its specifications, including requiring a Certificate of Analysis for each incoming shipment of vitamin and trace mineral premixes.
In December 2018, FDA issued an alert to pet owners regarding a series of dry dog food recalls for excessive vitamin D levels. As reported by Food Safety News, FDA received a total of six dog illness reports associated with the recalled products.
The dry dog foods were manufactured by Sunshine Mills and sold under several brand names.
An ordering error by a Sunshine employee caused the wrong Vitamin D ingredient to be shipped to the company.
According to FDA, Sunshine did not follow its own written procedures for receiving ingredients. The company neither obtained a Certificate of Analysis for the ingredient, nor conducted its own testing to determine whether the Vitamin D it received was the correct concentration for use in dog food.
The error resulted in a level of Vitamin D in the finished dog foods of as much as 70 times the target amount.
Sunshine was notified on October 23, 2018 about a consumer complaint reporting an ill dog that had eaten one of the company’s dry dog foods. Sunshine investigated the complaint and concluded on November 2nd that it was valid.
FDA requires validated complaints to be reported within 24 hours; however, Sunshine did not submit a ‘reportable food report to FDA until six days later, on November 8, 2018.
Although the sources of elevated vitamin D were different in these two situations, the course of events was similar.
- Both Hill’s and Sunshine took delivery of an ingredient that was substantially higher in vitamin D than specified for the purpose.
- Both Hill’s and Sunshine had written procedures in place for receiving raw materials, and these procedures mandated testing for vitamin D concentration.
- Neither Hill’s nor Sunshine carried out the lab analysis mandated in their written procedures.
- Neither Hill’s nor Sunshine required a Certificate of Analysis for their Vitamin D ingredient or premixes.
- Had Hill’s and Sunshine followed their own written procedures, the incorrect vitamin D ingredient concentration would have been found before the ingredient was used.
FDA’s investigation into Hill’s is ongoing, according to an agency spokesperson.
FDA offers the following information regarding vitamin D toxicity to pet owners and veterinarians.
- If your pet is having symptoms of vitamin D toxicity, contact a veterinarian immediately. Provide a full diet history to your veterinarian. You may find it helpful to take a picture of the pet food label, including the lot number.
- Don’t feed the recalled products to your pets or any other animal. Contact the company listed on the package for further instructions or throw the products away in a way that children, pets and wildlife cannot access them.
- Consumers can report suspected illness to the FDA electronically through the Safety Reporting Portal or by calling your state’s FDA Consumer Complaint Coordinators. It’s most helpful if you can work with your veterinarian to submit your pet’s medical records as part of your report. For an explanation of the information and level of detail that would be helpful to include in a complaint to the FDA, please see How to Report a Pet Food Complaint.
- The FDA encourages veterinarians treating vitamin D toxicity to ask their clients for a diet history. We also welcome case reports, especially those confirmed through diagnostics. You can submit these reports electronically through the Safety Reporting Portal or by calling your state’s FDA Consumer Complaint Coordinators. For an explanation of the information and level of detail that would be helpful to include in a complaint to the FDA, please see How to Report a Pet Food Complaint.
- Veterinarians should also be aware that vitamin D toxicity may present as hypercalcemia, similar to dogs that have consumed rodenticide. In these cases, FDA suggests that veterinarians confirm diet history to verify whether the dog has been eating any of the recalled products.
(To sign up for a free subscription to Food Safety News, click here.)
]]>The recalled product may contain translucent yellow pieces of rubber with a blue backing, which could present a choking hazard to cats.
The recall is limited to:
 Muse wet cat food Natural Chicken Recipe in Gravy, 3-oz cans:- Best by APR2020; Production code (first 8 characters) 80941162; UPC 38100 17199
Muse wet cat food Natural Chicken Recipe in Gravy, 3-oz cans:- Best by APR2020; Production code (first 8 characters) 80941162; UPC 38100 17199
The recalled products were distributed nationwide through pet specialty and e-commerce retailers.
The company initiated the recall after receiving complaints from pet owners who found the rubber pieces in the product.
Nestlé has not received any reports of injury or illness to date and has made changes to its process to avoid a repeat of the problem.
Consumers with questions regarding this recall may contact the company at 1-800-982-3885, 24 hours a day, 7 days a week.
Nestlé Purina Petcare is the St. Louis, MO-based subsidiary of Nestlé. It produces and markets pet food, treats and litter. Some of its pet food brands include Purina Pro Plan, Purina Dog Chow, Friskies, Beneful and Purina ONE
(To sign up for a free subscription to Food Safety News, click here.)
]]>The recall impacts Hill’s customers in at least 78 countries, according to information posted on Hill’s own websites, and the European Union’s Rapid Alert System for Food and Feed (RASFF).
An updated list of recalled products sorted by country is available at eFoodAlert.
Hill’s expanded its recall after FDA requested that the company test the vitamin D levels in additional products that were not part of the original recall. Testing leading up to both the January 31st and March 20th recalls revealed excessive, potentially toxic amounts of vitamin D, according to FDA.
All of the recalled products were manufactured using the same vitamin premix from a single U.S.-based supplier, according to Hill’s.
Although several hundred pet owners have posted complaints on the Hill’s Facebook page in response to the recall notice, there is no official tally of the number of affected pets.
In response to a question from Food Safety News, a spokesperson for FDA offered the following statement:
“The FDA has received a number of reports since the first Hill’s recall press release was issued on January 31, 2019. We are in the process of verifying the details of the complaints and it would be premature to release a number until the cases have been vetted to ensure they are all related to recalled product and are indeed cases of vitamin D toxicity.”
According to the FDA, dogs suffering from vitamin D toxicity may vomit, have little appetite, drink and urinate more, drool excessively, and/or lose weight. The severity of the symptoms and the speed of onset depends on the concentration of vitamin D ingested.
A dog exhibiting these symptoms should be seen by a veterinarian immediately.
Duncan’s story
Duncan was a 13-year old lascho bichon, a service dog trained for seizure alert, and Kelly’s constant companion.
Kelly and Duncan divided the year between their homes in Michigan and Florida. They walked together, rode golf carts together as many as four times a day.
Twelve years ago, as Kelly told Food Safety News, Duncan suffered from pancreatitis and was prescribed Hill’s Science Diet by his veterinarian. He ate Hill’s I/D and Z/D wet and dry foods.
In early January, Duncan started to vomit white foam, had excessive thirst and urination and was lethargic. The next morning, he awakened with tremors.
Over the next three days, during which Kelly brought Duncan to the veterinarian three times, Duncan lost excessive weight and his condition deteriorated.
Three weeks after Duncan’s death, Kelly read about the Hill’s recall. She contacted the company on February 6, and was offered compensation consisting of $10.00 in coupons for the purchase of Hill’s pet food.
Because Duncan died weeks before the recall was announced, there was no necropsy and no suspicion at the time that his death was due to vitamin D toxicity.
When asked by Food Safety News what message she wished to share with other pet parents, Kelly replied:
“Hill’s claims they subject all of their food to extensive and repeated testing. Yet, now they have admitted that they sold food contaminated with Vitamin D. Obviously, Hills did NOT have testing and quality controls in place to check the food before they put it on the shelves for sale. Also, they dragged their feet issued the three waves of the recall, with the last wave coming on March 20th. During that delay, additional pets were fed this poisoned food and have died. And, the recalls still don’t include all of the contaminated food. Why would anyone trust them now? Go to the Hills Pet Nutrition Facebook page and read the thousands of comments underneath the two recall notices from angry pet owners.”
Kelly has set up a Facebook Group, Saving Pets One Pet @ A Time, in Duncan’s memory where pet owners can comment on their experiences and share information about nutritious pet food options.
What pet owners should do
- If your pet is exhibiting symptoms of vitamin D toxicity, contact a veterinarian immediately. Provide a full diet history to your veterinarian. You may find it helpful to take a picture of the pet food label, including the lot number.
- Do not feed the recalled product to your pets. When discarding recalled products, make sure that they cannot be accessed by children, pets or wildlife. * Pet owners can report suspected illness to the FDA electronically through the Safety Reporting Portal or by calling your state’s FDA Consumer Complaint Coordinators. It’s most helpful if you can work with your veterinarian to submit your pet’s medical records as part of your report. For an explanation of the information and level of detail that would be helpful to include in a complaint to the FDA, please see How to Report a Pet Food Complaint.
What veterinarians should do
- Ask your clients for a diet history if you suspect vitamin D toxicity, which may present as hypercalcemia.
- Do not sell the recalled foods to your clients, and contact the manufacturer for further instructions. The FDA also encourages veterinarians to contact clients who have purchased recalled products, if they have the means to do so (such as through medical records or sales receipts).
- FDA welcomes case reports, especially those confirmed through diagnostics. You can submit these reports electronically through the Safety Reporting Portal or by calling your state’s FDA Consumer Complaint Coordinators. For an explanation of the information and level of detail that would be helpful to include in a complaint to the FDA, please see How to Report a Pet Food Complaint.
(To sign up for a free subscription to Food Safety News, click here.)
]]>In some cases, more than one pet in a household was affected.
In July 2018, FDA announced that it had begun investigating reports of DCM in pets who were fed certain pet foods containing high proportions of peas, lentils, pulses and/or potatoes. Many of the implicated pet foods are advertised as “grain-free.” Most of the reports, 276 out of 300, were received following the FDA’s announcement.
The investigation update does not include data from December 2018 or January 2019, as the partial government shutdown prevented FDA from continuing its investigation during that time period.
DCM is a recognized genetic condition in some dog breeds, including doberman pinschers, great danes, and Irish wolfhounds. It also has been reported in cocker spaniels.
Animals suffering from DCM develop an enlarged heart and may display symptoms such as decreased energy, cough, difficulty breathing and episodes of collapse. If caught early, the condition can be partially reversed with appropriate treatment and diet modifications.
The current spate of DCM reports is not limited to dog breeds known to have a genetic predisposition to the disease, but includes the following breeds, in descending order by frequency of reports: golden retrievers, mixed breed dogs, Labrador retrievers, great danes, Australian shepherds, German shepherds, pit bulls, boxers, doberman pinschers, mastiffs, American cocker spaniels, standard poodles, Shetland sheepdogs, weimaraners, French bulldogs, Australian cattle dogs, Boston terriers, bulldogs, samoyeds, and shih-tsus.
Other breeds with more than one case report include: Afghan hound, beagle, dalmatian, English springer spaniel, flat-coated retriever, unspecified hounds, Maltese, miniature schnauzer, pomeranian, Portuguese water dog, pug, unspecified retriever, Rhodesian ridgeback, rottweiler, saluki, vizsla, and Yorkshire terrier.
Ages of affected dogs range between less than 6 months to 16 years. Dogs suffering from DCM weighed between 8 pounds and 212 pounds. More male dogs than female dogs have been affected.
In contrast, genetically related DCM tends to involve middle-aged to older aged male dogs of large and giant breeds.
The majority of cases reported to FDA, 269 of 325, involved dogs fed dry foods, approximately 90 percent of which were reported to be “grain-free.” Although most of the diets included an animal protein, such as fish, eggs, lamb or chicken, no single source predominated.
Since beginning its investigation, FDA’s Veterinary Laboratory Investigation and Response Network (Vet-LIRN), has tested various products for minerals and metals including calcium, magnesium, phosphorus, iron, cobalt, copper, zinc, selenium and iodine. They also tested for amino acids, including taurine, cysteine and methionine. Cysteine and methionine are required for the body to manufacture taurine.
In addition, Vet-LIRN has tested both grain-free and grain-containing products for protein, fat, moisture, crude fiber, total dietary fiber, soluble fiber, insoluble fiber, total starch, and resistant starch. Grain-free products were higher in fiber and lower in starch than grain-containing products. Otherwise, there was very little difference between the grain-free and grain-containing products.
FDA has received lab reports, diagnostic records such as echocardiograms, and necropsy reports from some of the affected dogs. In addition, the agency is collaborating with Chesapeake Veterinary Cardiology Associates (CVCA) on a prospective study of DCM-diagnosed dogs.
CVCA is collecting medical records, owner interviews, diagnostic samples from each of the diagnosed animals, and is archiving feces and DNA samples for possible future testing.
FDA also has been working with Drs. Lisa Freeman of Tufts University, Joshua Stern of the University of California-Davis, and Darcy Adin of the University of Florida. Stern has been studying the increases in DCM cases in golden retrievers. Many of these cases are associated with taurine deficiency.
The FDA reported it is not aware of similar DCM illness reports or investigations in other countries, according to a spokesperson for the agency, adding that FDA would welcome the opportunity to collaborate with international counterparts on diet-related DCM.
A number of researchers in Canada and the United States, led by W.D. Mansilla of the University of Guelph in Ontario, Canada, recently issued a report on the association between DCM and pulse ingredients in pet food. They suggested L-carnitine deficiency as another possible avenue of investigation into the cause of non-hereditary DCM.
L-carnitine, an amino acid, occurs naturally in animal protein, but is absent from plant protein. It is present at highest concentration in red meats such as lamb and beef, and at a lower level in pork, poultry and fish.
When asked whether FDA was examining the possible effect of L-carnitine on the development of DCM, an agency spokesperson said the agency and its investigative partners “. . . are considering or open to considering any science and evidence-backed theory.”
Guidance to pet owners and veterinarians
If a dog is showing possible signs of DCM or other heart conditions, including decreased energy, cough, difficulty breathing and episodes of collapse, contact your veterinarian as soon as possible. If the symptoms are severe and your veterinarian is not available, you may need to seek emergency veterinary care. Be prepared to provide your veterinarian with a thorough dietary history, including all the foods (including treats) the dog has eaten.
The FDA encourages veterinary professionals to report well-documented cases of DCM in dogs suspected of having a link to diet by using the electronic Safety Reporting Portal or calling their state’s FDA Consumer Complaint Coordinators.
The more information veterinarians provide, particularly feeding history, medical records, and diagnostic testing, the better. Detailed instructions can be found on “How to Report a Pet Food Complaint.” Technical veterinary information that may aid veterinarians can be found in the agency’s Vet-LIRN Update – February 2019.
(To sign up for a free subscription to Food Safety News, click here.)
]]>Pet owners have filed a total of three separate civil lawsuits in federal court against Hill’s Pet Nutrition Inc. following the company’s recent admission that some of its pet foods contained excessive levels of Vitamin D. The consumers are seeking class action status.
The named plaintiffs in the three lawsuits allege that their dogs became ill as a result of elevated vitamin D in the pet foods. Four of the sick dogs either died or had to be euthanized. The pet owner plaintiffs live in Florida, New York, California and North Carolina.
The lawsuits allege negligence, breach of express and implied warranty, strict product liability, failure to warn, unjust enrichment, and unfair and deceptive trade practices on the part of the company.
In addition, plaintiffs in one of the lawsuits accused Hill’s of “excessive and unwarranted delay” in notifying consumers and regulatory agencies of the Vitamin D toxicity issue.
According to a spokesperson for the Food and Drug Administration, Hill’s alerted the agency to a Vitamin D-related complaint on Jan. 30 this year. The company announced a recall of specific batches of 25 canned pet foods the following day.
The affected batch codes suggest the recalled products were likely manufactured during the time period of September 2018 through December 2018.
FDA has received a number of pet illness reports since the Jan. 31 recall notice. The agency is in the process of verifying the details of each complaint to determine which reports are related to the recalled products, and whether they are cases of Vitamin D toxicity.
The recalled products were distributed nationwide in the U.S. and exported to at least 57 countries. In its recall notice, Hill’s advised consumers to refer to the company’s website for their country for a list of recalled products.
Recalled products were sold in Albania, Andorra, Austria, Australia, Belarus, Belgium, Bosnia and Herzegovina, Bulgaria, Canada, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hong Kong, Hungary, Iceland, Indonesia, Ireland, Italy, Japan, Kosovo, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Mexico, Moldova, Monaco, Montenegro, Netherlands, New Zealand, Norway, Poland, Portugal, Republic of North Macedonia, Romania, Russia, San Marino, Serbia, Singapore, Slovakia, Slovenia, South Africa, South Korea, Spain, Sweden, Switzerland, Turkey, Ukraine, United Kingdom, USA, Vatican City
Using information obtained from Hill’s websites around the world, eFoodAlert posted a list of recalled products and batch codes, sorted by country.
In November and December 2018, nine companies recalled several dry pet food products because of elevated Vitamin D issues. All of the foods were produced and packaged by a common contract manufacturer, identified by several of the companies as Sunshine Mills Inc.
FDA has not found any connection between the source of Vitamin D used to manufacture the recalled dry dog foods and the Vitamin D used in the Hill’s canned dog foods, according to an agency spokesperson.
What pet owners and veterinarians should know
Symptoms of Vitamin D toxicity may include vomiting, loss of appetite, increased thirst and urination, excessive drooling and/or weight loss. FDA urges dog owners who observe these symptoms in their pets to contact a veterinarian immediately.
Pet owners should be prepared to furnish a full diet history to the veterinarian, and to document the pet food label and lot number. Any leftover food should be retained in its original packaging in case testing is required.
FDA encourages veterinarians to submit case reports involving vitamin D toxicity diagnoses associated with pet food.
Consumers and veterinarians can submit complaints and case details to the FDA through the Safety Reporting Portal or by calling their local FDA Consumer Complaint Coordinators. For additional information on submitting a pet food complaint, see How to Report a Pet Food Complaint.
(To sign up for a free subscription to Food Safety News, click here.)
]]>The recall is being carried out in cooperation with the U.S. Food and Drug Administration (FDA). Hill’s own investigation confirmed the presence of elevated levels of vitamin D due to a supplier error.
Depending on the level of vitamin D and the length of exposure, dogs that have ingested excessive vitamin D may exhibit symptoms such as vomiting, loss of appetite, increased thirst, increased urination, excessive drooling, and weight loss.
When consumed at very high levels, vitamin D ingestion can lead to serious conditions, including kidney dysfunction. In most cases, the symptoms are reversible and complete recovery occurs after discontinuation of feeding.
The following recalled canned food products were distributed through retail pet stores and veterinary clinics across the United States.
- Hill’s® Prescription Diet® c/d® Multicare Canine Chicken & Vegetable Stew, 12.5oz:- SKU #3384. Lot codes 102020T10, 102020T25
- Hill’s® Prescription Diet® i/d® Canine Chicken & Vegetable Stew, 12.5oz:- SKU #3389. Lot codes 102020T04, 102020T10, 102020T19
- Hill’s® Prescription Diet® i/d® Canine Chicken & Vegetable Stew, 5.5oz:- SKU #3390, Lot codes 102020T11, 112020T23, 122020T07
- Hill’s® Prescription Diet® z/d® Canine, 5.5oz:- SKU #5403, Lot codes 102020T17, 112020T22
- Hill’s® Prescription Diet® g/d® Canine, 13oz:- SKU #7006, Lot code 112020T19
- Hill’s® Prescription Diet® i/d® Canine, 13oz:- SKU #7008, Lot codes 092020T30, 102020T07, 102020T11, 112020T22
- Hill’s® Prescription Diet® j/d® Canine, 13oz:- SKU #7009, Lot code 112020T20
- Hill’s® Prescription Diet® k/d® Canine, 13oz:- SKU #7010, Lot code 102020T10
- Hill’s® Prescription Diet® w/d® Canine, 13oz:- SKU #7017, Lot codes 092020T30, 102020T11
- Hill’s® Prescription Diet® z/d® Canine, 13oz:- SKU #7018, Lot codes 102020T04, 112020T22
- Hill’s® Prescription Diet® Metabolic + Mobility Canine Vegetable & Tuna Stew, 12.5oz:- SKU #10086, Lot codes 102020T05, 102020T26
- Hill’s® Prescription Diet® w/d® Canine Vegetable & Chicken Stew, 12.5oz:- SKU #10129, Lot codes 102020T04, 102020T21
- Hill’s® Prescription Diet® i/d® Low Fat Canine Rice, Vegetable & Chicken Stew, 12.5oz:- SKU #10423, Lot codes 102020T17, 102020T19, 112020T04
- Hill’s® Prescription Diet® Derm Defense® Canine Chicken & Vegetable Stew, 12.5oz:- SKU #10509, Lot code 102020T05
- Hill’s® Science Diet® Adult 7+ Small & Toy Breed Chicken & Barley Entrée Dog Food, 5.8oz:- SKU #4969, Lot code 102020T18
- Hill’s® Science Diet® Puppy Chicken & Barley Entrée, 13oz:- SKU #7036; Lot code 102020T12
- Hill’s® Science Diet® Adult Chicken & Barley Entrée Dog Food, 13oz:- SKU #7037, Lot codes 102020T13, 112020T23
- Hill’s® Science Diet® Adult Turkey & Barley Dog Food, 13oz:- SKU #7038, Lot code 102020T06
- Hill’s® Science Diet® Adult Chicken & Beef Entrée Dog Food, 13oz:- SKU #7040, Lot code 102020T13
- Hill’s® Science Diet® Adult Light with Liver Dog Food, 13oz:- SKU #7048, Lot code 112020T19
- Hill’s® Science Diet® Adult 7+ Chicken & Barley Entrée Dog Food, 13oz:- SKU #7055, Lot codes 092020T31, 102020T13
- *Hill’s® Science Diet® Adult 7+ Beef & Barley Entrée Dog Food, 13oz:- SKU #7056, Lot codes 092020T31, 112020T20, 112020T24
- Hill’s® Science Diet® Adult 7+ Turkey & Barley Entrée, 13oz:- SKU #7057, Lot code 112020T19
- Hill’s® Science Diet® Adult 7+ Healthy Cuisine Braised Beef, Carrots & Peas Stew dog food, 12.5oz:- SKU #10452, Lot codes 102020T14, 102020T21
No dry foods, cat foods or treats are affected by this recall. Pet owners who purchased any of the recalled products should discontinue feeding them to their pets, and should dispose of the products immediately.
In addition to having been distributed nationwide across the United States, some of the recalled products were exported to other countries. Hill’s encourages customers outside the USA to consult their own country’s Hill’s website for more information.
In December 2018, FDA alerted pet owners to potentially toxic levels of vitamin D in several dry pet food brands. The affected products were manufactured by a common contract manufacturer.
The current recall is the first to involve canned pet food.
Company Information can also be found at www.hillspet.com/productlist
]]>On Jan. 23, 2019, the U.S. Food and Drug Administration (FDA) cautioned pet owners not to feed one lot of Hare Today Gone Tomorrow Ground Chicken/Bones/Organs pet food to their pets after the agency found Salmonella and Listeria monocytogenes in samples of the product.
FDA acted in response to a consumer complaint about a kitten that became sick with Salmonella after eating the company’s product.
According to the FDA alert, the specific batch of product consumed by the kitten was no longer available for testing. FDA collected samples from a different bunch of the same product.
The strain of Salmonella found in the batch of product analyzed by FDA was different from the strain recovered from the sick kitten.
When advised by FDA of the test results, Hare Today’s management refused to initiate a recall of the contaminated product.
In a Facebook post, the company supported its decision by claiming that there were no government seals on the samples when they were received by the lab, that FDA could not provide Hare Today with information on the temperature of the sample at the time of testing and, therefore, that the products “…were compromised and any results are null and void.”
The company also alleged that FDA is unfairly targeting raw pet food manufacturers.
According to an FDA spokesperson, sampling was carried out under the requirements set out in the agency’s Investigations Operations Manual. The inspector obtained ten random unopened packages from a single batch of frozen product (processing date 12.04.2018). Each box was placed into a separate container, which was taped shut.
The ten individual packages, plus two controls, were placed in a cooler for shipment to the laboratory. The boxes were sent frozen at the time of sampling and received frozen in the FDA lab.
FDA always prefers to test the specific lot associated with a case, the agency spokesperson told Food Safety News. However, the consumer did not have any opened or unopened product left from the suspect batch.
Due to the length of time that elapsed between the feeding of the kitten and the submission of the complaint and associated records to FDA, none of the implicated batches was available from the manufacturer either.
The feces sample from the sick kitten was collected in May 2018, according to the sample record archived in the National Center for Biotechnology Information database.
Hare Today sells all of its products through direct orders placed with the company via its website and does not use third-party distributors or retailers.
The Hare Today allegations echo similar statements made by Lystn, dba Answers Pet Food earlier this month.
After the Nebraska Department of Agriculture (NDA) recovered Salmonella from a sample of A+ Answers Pet Food, the company accused the state of mishandling the sample.
A spokesperson for the NDA refuted the company’s allegations, stating that all “chain of custody” procedures had been followed, that the sample was shipped in dry ice and arrived frozen in the lab, and that all procedures had been followed to prevent cross-contamination.
The presence of Salmonella in a pet food represents a hazard to both people and pets, regardless of whether the food is raw, dry, or cooked.
People infected with Salmonella may suffer from diarrhea, abdominal cramps, and fever. Young children, the elderly, and individuals with weakened immune systems are susceptible to more severe symptoms, including dehydration, and may require hospitalization.
Infected pets may exhibit symptoms including vomiting, diarrhea (sometimes bloody), fever and loss of appetite or decreased activity. Pets that are infected can shed Salmonella in their feces even without showing any evidence of being sick.
FDA recommends that people who think their pets have become ill after consuming contaminated pet food should first contact their veterinarians. Veterinarians who wish to have pets tested for Salmonella may do so through the Vet-LIRN Network if the pet is from a household with a person infected with Salmonella. Veterinarians who wish to have pets tested for other pathogens when there is an associated human case may also contact Vet-LIRN.
The FDA encourages consumers to report complaints about this and other pet food products electronically through the Safety Reporting Portal or by calling their state’s FDA Consumer Complaint Coordinators.
(To sign up for a free subscription to Food Safety News, click here.)
Public health officials discovered during their investigation that Woody’s Pet Deli raw turkey pet food was fed regularly to a pet in the household of the infected individual. The pet tested positive for a different strain of Salmonella.
Woody’s is a small Minnesota-based chain of pet food shops, with locations in Minneapolis, St. Paul, and Woodbury.
The recalled product was sold in 5-pound plastic containers, identified as “Woody’s Pet Food Deli Raw Free Range Turkey,” and bearing one of the following Use By dates: 01/10/20, 01/12/20, 01/15/20.
The U.S. Centers for Disease Control and Prevention is investigating an ongoing multi-state outbreak of Salmonella Reading infections linked to raw turkey products from multiple sources. The outbreak has been in progress since November 2017. As of Dec. 18, 2018, there had been 216 cases of Salmonella Reading illnesses reported in 38 states. One person has died and 84 have been hospitalized.
The Public Health Agency of Canada (PHAC) has been tracking 33 Salmonella illnesses in six provinces. Those patients are linked to raw turkey and raw chicken products. The same outbreak strain of Salmonella Reading has been recovered from both Canadian and U.S. patients.
No single source or supplier in either the United States or Canada of raw turkey products or of live turkeys has been identified that could account for the whole outbreak.
In November 2018 and again in December 2018, Jennie-O-Turkey Store Sales in Wisconsin and Minnesota respectively recalled nearly 128 tons of raw ground turkey products that were associated with the Salmonella Reading outbreak.
This is the second pet food recall linked to the Salmonella Reading outbreak. In February 2018, Raws for Paws recalled about 4,000 pounds of ground turkey pet food after two children were infected with Salmonella Reading. The outbreak strain was recovered from samples of the Raws for Paws food fed to pets in the household where the children lived. One of the two children was hospitalized with osteomyelitis.
Symptoms of Salmonella infection in people usually include diarrhea, abdominal pain and fever. Infected pets may experience diarrhea, fever and vomiting, or may be without symptoms. Even asymptomatic pets may shed Salmonella in their feces, spreading the infection in the environment.
Individuals who have purchased the recalled Woody’s product should throw it out or return it for a full refund. Consumers with questions should telephone their Woody’s store or contact the company by email at [email protected].
(To sign up for a free subscription to Food Safety News, click here.)
]]>Lystn, the manufacturer of Answers Pet Foods, offers a range of raw pet foods “enhanced” with kombucha, raw cultured whey, cultured raw goat’s milk and kefir. According to the company, the probiotic bacteria in the fermented ingredients offer protection against Salmonella.
Lystn describes this approach on its website as “safety through inhibition” and claims the fermentation process to be the “most natural and effective way” to make their products “as safe and healthy as possible.”
In 2009, A.R. Hoyle and co-workers reported that lactic acid bacteria could decrease the numbers of Salmonella and E. coli O157:H7 in ground beef during storage. However, several studies have shown that use of lactic acid bacteria is most effective when incorporated into a combined strategy employing other complementary treatments.
On Dec. 10, 2018, the Nebraska Department of Agriculture (NDA) obtained a random sample of A+ Answers Straight Beef Formula 4lb. Pounder for Dogs, Lot 2018 02/08 20. On or about Dec. 20, NDA advised both Lystn and the FDA that it had found Salmonella in the sample.
Lystn requested and received from the NDA a split sample of the implicated lot, and confirmed the presence of Salmonella in this sample, according to the company’s statement. A second split sample provided to Lystn by NDA at the company’s request produced a negative result.
In its press release, Lystn expressed its “belief” that the initial split sample provided by the NDA “. . . may have been cross contaminated in the lab, transport or elsewhere and should not be considered a representative sample.” The company offered no evidence to support the assertion.
Lystn also disputed the appropriateness of FDA’s zero tolerance policy for Salmonella in raw pet food.
No FDA spokesperson was available for comment because of the partial government shutdown.
The NDA inspector and lab personnel used appropriate chain-of-custody procedures for handling the pet food sample, according to a spokesperson for the state. The sample was handled and analyzed in a Biosafety Level 2 (BSL-2) lab, under full compliance with all sanitation and handling procedures.
No other testing was performed in the lab at the same time. Access to a BSL-2 lab is restricted while testing is being conducted in order to limit any possible risk of inadvertent contamination of the sample, the environment, or personnel, according to the U.S. Centers for Disease Control and Prevention (CDC) website.
The lab personnel used both positive and negative controls as part of their testing protocol, and submitted the purified Salmonella culture to the Nebraska Public Health Laboratory (NPHL) for molecular testing. NPHL identified the culture as Salmonella Cerro. The positive control used as part of the testing procedure was Salmonella Arizonae.
According to Lystn, the company stopped distribution of the lot in question, and the product was removed from retail store shelves within the state of Nebraska.
Although Lystn asserted in its Jan. 15 statement that the product was not recalled, the company’s action met FDA’s definition of a product recall, which is “. . . a firm’s removal or correction of a marketed product that the FDA considers to be in violation of the laws it administers and against which the agency would initiate legal action.”
The company chose not to implement a nationwide recall of the contaminated production lot, as the product was only tested by NDA and not by FDA, according to the Lynst statement.
Lystn’s customers are welcome to return any unused portion of the affected product for full refund, according to the company’s press release, which stated “. . . if a customer is uncomfortable with an ANSWERS’ product, they may return it, or any unused portion, to the place of purchase for a full refund. The Straight Beef Formula 4lb. Pounder for Dogs product comes in a cardboard milk carton box marked with lot code 2018 and a Best Use By Date (BUBD) of 02/08 20 sticker on the carton.”
The implicated lot was distributed from Aug. 17, 2018 to Sept. 14, 2018, and sold through retail stores within the United States.
A Lystn spokesperson was unable to release further details at this time, stating that the company was in the process of completing its investigation on the products from NDA as well as working with FDA.
(To sign up for a free subscription to Food Safety News, click here.)
]]>The most dangerous time of year for pets in the United States is right now: the period leading up to, and including, the Christmas and New Year holidays. This is the time of year that presents both pets and people with a plethora of opportunities to be “indiscreet” eaters.
It is tempting to offer a pet a special holiday treat: an unfamiliar food, a chance to lick raw cake batter from a mixing bowl, or a dish of leftovers from the banquet table. It is easy to overlook the risks posed by Christmas decorations, snack foods, candies, and candles.
Some of these temptations are equally hazardous to the humans in our households. Both flour and raw eggs are potential sources of Salmonella, and raw flour may contain E. coli bacteria.
Leftover food that sits out for hours at room temperature during and after holiday meals are breeding grounds for toxin-producing bacteria, including Staphylococcus aureus (staph) and Clostridium perfringens. Both of these pathogens can make pets and people sick with acute nausea, vomiting and diarrhea.
Cats, especially, are sensitive to staph toxins. In fact, the earliest test for the presence of this toxin in a food sample was the aptly named Kitten Test, in which a portion of the food was fed to kittens. An episode of vomiting within a few hours confirmed the presence of the toxin.
Clostridium perfringens can be deadly for dogs. A 2012 article published in the Canadian Veterinary Journal reported on the death due to acute bloody diarrhea, of a two-year old Pomeranian show dog that had appeared perfectly healthy the day before. Large numbers of Clostridium perfringens were found in the dog’s intestinal tract on necropsy.
Following these tips will help you to keep your pets and family members safe during the holidays.
DO refrigerate leftovers promptly.
DO handle raw meat and poultry as though contains Salmonella and Campylobacter, because it probably is contaminated with one or both of these pathogens.
DO place all chocolate, candies and plants out of reach of pets.
DO brush up on practices for the safe handling of foods.
DO visit the ASPCA Animal Poison Control Center website to familiarize yourself with foods, plants and household products that may be toxic to your pets.
DON’T allow your pets or children to sample raw cake batter, raw cookie dough, raw pie crust or any other raw baked goods.
DON’T offer unusual food to your pets. This is not the time of year to experiment.
DON’T feed your pets raw poultry.
DON’T offer cooked bones of any type to your dog. Cooking makes bones more brittle and they may splinter and injure your pet.
DON’T set aside safe food handling practices in your hurry to prepare for the deluge of holiday guests.
(To sign up for a free subscription to Food Safety News, click here.)
]]>From August 2007 through Dec. 31, 2015, the number of illness complaints linked to jerky pet treats included more than 6,200 dogs, 26 cats, and three people. More than 1,140 of the dogs died.
In a Grand Rounds webinar in recent days, Dr. Lee Anne Palmer of FDA’s Center for Veterinary Medicine (CVM) summarized the outcome of the agency’s decade-long investigation.
The first hint of trouble appeared in August 2007, when bloggers reported the removal of chicken jerky pet treats from the shelves of a major retailer due to traces of melamine in the treats.
In September 2007, the American Veterinary Medical Association (AVMA) alerted its members to reports of an acquired form of Fanconi syndrome in dogs. Fanconi syndrome is a potentially fatal disorder of the urinary tract.
Later that same month, FDA advised pet owners of a “… potential association between development of illness in dogs and the consumption of chicken jerky products…” The agency reported having received in excess of 70 complaints involving more than 95 dogs. In addition, FDA received information from Banfield, The Pet Hospital suggesting an association between exposure to chicken jerky products and signs of gastrointestinal illness in dogs, including vomiting and diarrhea.
FDA investigated the reports, but was unsuccessful in finding the cause of the illnesses.
In late 2013, FDA issued a comprehensive update on its investigation, including a fact sheet for pet owners and a “Dear Veterinarian” letter requesting specific clinical data. The update triggered an immediate, massive increase in illness reports.
In 2014, FDA enlisted the help of the U.S. Centers for Disease Control and Prevention to design and run a case control study. Scientists from CVM and CDC enrolled 95 affected dogs from 31 states for the study, matching them with 261 controls.
The case control study, first of its kind for a pet illness investigation, determined that the illnesses were highly associated with consumption of pet jerky treats from China. There was also some association with jerky treats from the United States.
Ill dogs were more likely to be female, and small breeds were more likely to be affected than larger breeds. No other exposures were associated with the pet illnesses.
Since 2007, FDA has received reports of pet illness related to jerky treats from all 50 U.S. states, most Canadian provinces, and several other countries, including the United Kingdom, Germany, Switzerland, and Singapore, according to Palmer.
In 2007, the only way for consumers or veterinarians to report pet illnesses to FDA was by contacting a Consumer Complaint Coordinator by telephone. There was no system in place to coordinate or collate the reports. Even today, as Palmer observed, there is no CDC for pets.
In May 2010, CVM introduced the Safety Reporting Portal, an on-line form that can be used by consumers and veterinarians to report illnesses linked to pet foods, treats or medications.
In January 2011, CVM combined the two parallel information streams into a single database and initiated a weekly review of the data to identify trends.
FDA obtained funding in 2010 to establish the Veterinary Laboratory Response Network (Vet-LIRN), a collaborative network of government and university laboratories that form the backbone of FDA’s ability to document, investigate, and diagnose animal feed, pet food, and animal drug-related illnesses. This powerful resource was activated in August 2011 and has now grown to include 43 participating laboratories.
While FDA’s efforts did not establish a single root cause of pet illnesses linked to jerky pet treats, the investigations revealed a number of issues resulting in recalls or import alerts for various problems, including:
- Salmonella contamination
- Residues of antibiotic and antiviral agents
- Mislabelling
- Melamine traces
- Excessive levels of glycerin
The cumulative effect of these recalls and import alerts produced, over time, a significant reduction in the number pet illnesses associated with pet jerky treats.
According to a spokesperson for FDA, the number of reported illnesses associated with jerky pet treats has waned in recent years and returned to baseline levels. The agency is dialing back its use of investigative resources on jerky pet treats to focus on other types of pet food product complaints.
FDA expects to publish a final update on its website summarizing the investigation at a future date.
Although a single root cause of the treat-related illnesses was never found, the analytical and tracking tools developed during the treat investigation have helped CVM to more quickly identify and respond to several other pet food issues in recent years. These include:
- Pentobarbital contamination in canned dog foods
- Thiamine deficiency in cat food
- Vitamin D excess in dog food
- Thyroid gland contamination of canned dog food and domestic jerky treats
- Salmonella contamination of dry dog foods
- Listeria and Salmonella in raw pet foods
- Dilated cardiomyopathy and grain-free dog foods
Consumers and veterinarians who are concerned about a pet illness that appears to be linked to a pet food or pet treat should report the incident using FDA’s Safety Reporting Portal or by contacting the Consumer Complaint Coordinator for their district.
(To sign up for a free subscription to Food Safety News, click here.)
]]>The recalled pet food has the potential to contain an elevated level of vitamin D, which can produce symptoms of vomiting, loss of appetite, increased thirst, increased urination, excessive drooling, and weight loss in dogs. In extreme cases, ingestion of food containing elevated vitamin D may result in kidney failure.
On Dec. 3, the Food and Drug Administration issued a public alert about the potential for elevated vitamin D levels in several brands of dry pet foods produced by “a common contract manufacturer.” King Soopers and Kroger identified Sunshine Mills Inc. as the manufacturer in their recall notices.
FDA received two pet illness complaints in mid- to late October and initiated an investigation at that time, according to an agency spokesperson. In late October, FDA was alerted to a similar investigation being carried out by the state of Utah.
As of Dec. 3, the FDA has received a total of six dog illness reports associated with the recalled products. The investigation is ongoing, and the number of illnesses may change.
FDA, state and private lab tests have revealed that the pet food contained as much as 70 times the intended amount of vitamin D. Consumers are warned that these levels of vitamin D are potentially toxic to dogs and may lead to kidney failure and/or death.
The products recalled by King Soopers and Kroger include:
- Abound Chicken and Brown Rice Recipe Dog food, 4 lb. Best by November 1, 2018 through November 16, 2019; UPC 11110-83556
- Abound Chicken and Brown Rice Recipe Dog food, 14 lb. Best by November 1, 2018 through November 16, 2019; UPC 11110-83573
- Abound Chicken and Brown Rice Recipe Dog food, 24 lb. Best by November 1, 2018 through November 16, 2019; UPC 11110-89076
All three package sizes were sold in King Soopers and City Market stores in Colorado, Utah, New Mexico and Wyoming. The 4-pound size was sold in a single Kroger store at 2440 Bardstown Road in Louisville, KY.
FDA offers the following recommendations to pet owners and veterinarians.
- If your pet is having symptoms of vitamin D toxicity, contact a veterinarian immediately. Provide a full diet history to your veterinarian. You may find it helpful to take a picture of the pet food label, including the lot number.
- Don’t feed the recalled products to your pets or any other animal. Contact the company listed on the package for further instructions or throw the products away in a way that children, pets and wildlife cannot access them.
- Consumers can report suspected illness to the FDA electronically through the Safety Reporting Portal or by calling your state’s FDA Consumer Complaint Coordinators. It’s most helpful if you can work with your veterinarian to submit your pet’s medical records as part of your report. For an explanation of the information and level of detail that would be helpful to include in a complaint to the FDA, please see How to Report a Pet Food Complaint.
- The FDA encourages veterinarians treating vitamin D toxicity to ask their clients for a diet history. We also welcome case reports, especially those confirmed through diagnostics. You can submit these reports electronically through the Safety Reporting Portal or by calling your state’s FDA Consumer Complaint Coordinators. For an explanation of the information and level of detail that would be helpful to include in a complaint to the FDA, please see How to Report a Pet Food Complaint.
- Veterinarians should also be aware that vitamin D toxicity may present as hypercalcemia, similar to dogs that have consumed rodenticide. In these cases, FDA suggests that veterinarians confirm diet history to verify whether the dog has been eating any of the recalled products.
For additional coverage of the recent Sunshine pet food recalls, please see:
“Sunshine recalls three more dog food brands for excess vitamin D; distribution international”
“Sunshine Mills recalls additional dog foods for excess vitamin D”
(To sign up for a free subscription to Food Safety News, click here.)
]]>Ingestion of the barbiturate can cause drowsiness, dizziness, excitement, loss of balance, or nausea, or in extreme cases, possibly death of dogs and cats. Beef tallow is promoted as an inexpensive, palatable, and stable source of energy, with a nutritional profile “sufficient for safe use as a pet food ingredient,” according to an industry website, BeefTallow.com.
Champion, a Canadian pet food company whose U.S. production facility is in Auburn, KY, manufactures Acana and Orijen brands of dry dog food.
The company ceased manufacturing products that require beef tallow, and quarantined the three pentobarbital-contaminated lots of tallow. However, by the time the company learned of the problem, some of the tallow already had been used.
Champion initiated pentobarbital testing on the retained samples from the beginning, middle and end of the affected production lots, and retrieved finished product from its third-party distributors.
The retained samples tested for pentobarbital were found to be negative. Some of the finished products containing contaminated tallow were distributed to the store/consumer level, but no retail-level product recall was initiated.
On May 16 the Food and Drug Administration initiated an inspection of Champion’s facility, according to information contained in the agency’s Establishment Inspection Report (EIR), which was obtained by Food Safety News in response to a Freedom of Information Act request.
During the inspection, the company informed FDA that Champion was no longer doing business with the Pennsylvania supplier of beef tallow and consulted with the agency on how to properly dispose of the pentobarbital-contaminated tallow.
The name of Champion’s tallow supplier was blacked out in the EIR.
Champion advised FDA inspectors that it will require its new supplier of tallow to include test results for pentobarbital on Certificates of Analysis accompanying all shipments of the ingredient. FDA investigators appeared satisfied with Champion’s response to the contaminated tallow episode, classifying the outcome of the investigation as “No Action Indicated.”
Since the beginning of 2017, pentobarbital contamination has triggered the recall of canned pet foods manufactured by Evanger’s Dog and Cat Food Co. and Big Heart Brands.
The source of pentobarbital contamination in the Evanger’s products was never established.
Beef tallow was the apparent source of pentobarbital contamination in Big Heart Brands’ canned pet foods. According to information contained in a class action complaint filed against the manufacturer of Gravy Train and other canned dog foods, Big Heart’s tallow supplier was MOPAC, a Pennsylvania rendering facility belonging to JBS USA Holding Inc.
Big Heart Brands packs several popular brands of pet food that are distributed nationwide. Those brands include Gravy Train, Kibbles ’N Bits, Skippy and Ol’ Roy brands. Big Heart Pet Brands distributed the Gravy Train, Kibbles ’N Bits and Skippy dog food to retailers nationwide. Walmart Stores Inc. distributed the Ol’ Roy dog food.
(To sign up for a free subscription to Food Safety News, click here.)
]]>Consumers should stop feeding the products listed below. Dogs consuming elevated levels of Vitamin D could exhibit symptoms such as vomiting, loss of appetite, increased thirst, increased urination, excessive drooling, and weight loss.
The following products are included in the voluntary recalls, which are being conducted in cooperation with the U.S. Food and Drug Administration (FDA).
Lidl US / Sunshine Mills
Orlando brand Grain Free Chicken & Chickpea Superfood Recipe Dog Food:- Lidl product number 215662; Lot numbers TI1 3 Mar 2019, TB2 21 Mar 2019, TB3 21 Mar 2019, TA2 19 Apr 2019, TB1 15 May 2019, and TB2 15 May 2019; manufactured between March 3, 2018 and May 15, 2018
Natural Life Pet Products
Natural Life Chicken & Potato Dry Dog Food:- 17.5-lb bags; UPC 0-12344-08175-1; Best by dates December 4, 2019 through August 10, 2010
Lidl has removed the Orlando brand dog food from its stores.
The Natural Life product dog food was distributed to retail stores in Georgia, Florida, Alabama, North Carolina, South Carolina, Tennessee, Virginia, and California.
Both companies are asking customers who have purchased any of these products to discard the food or return it for a full refund.
Lidl customers who have questions about this recall should call the Lidl US Customer Care Hotline at 1-844-747-5435 (8 am-9 pm Eastern time 7 days a week).
Consumers with questions about the Natural Life recall may contact the company at (888) 279-9420 from 8 AM to 5 PM Central Standard time, Monday through Friday, or by email at [email protected] for more information.
]]>“Regulation is bad for business.”
It’s an all-too-common refrain, but not exclusively a modern one.
Most people, when asked what they know about the origin of the U.S. Food, Drug and Cosmetics Act, are likely to respond with either a shrug or a blank stare. A few may credit Upton Sinclair’s exposé of the horrendous practices in Chicago slaughterhouses, as described in his novel, “The Jungle.” Others may give credit to President Theodore Roosevelt, whose disgust at the food supplied to his troops in Cuba during the Spanish American War compelled him to support the campaign for safer food.
It would be a rare person, indeed, who would recognize the name of Dr. Harvey Washington Wiley. Yet, without Wiley’s persistence, the Pure Food and Drug Act of 1906 might never have been signed into law.
Before the turn of the 20th century, food adulteration was rife in the United States, and food labeling non-existent. Formaldehyde and other toxic chemicals were used to extend the shelf life of perishable commodities. Dyes derived from coal tar were incorporated into everything from candy to peas.
Unregulated, free enterprise prevailed. Embalmed beef, swill milk, and fake food were the order of the day.
In 1882, Wiley joined the USDA as chief chemist, a position he would hold for 30 years. The first 24 years of his stewardship were devoted to promoting the passage of a safe food law. The remaining six years were consumed with protecting the act from emasculation.
“The Poison Squad” is the story of Wiley’s lifelong crusade for safe food in the face of opposition from large swaths of the chemicals, dyes, and food processing industries.
Author Deborah Blum guides the reader through his complex and compelling saga with skill, and with a clear mastery of the subject matter. Consumers and legislators alike can learn from Blum’s insights on Wiley’s legacy.

Photo by Mark Bennington
About Deborah Blum
Deborah Blum is director of the Knight School Journalism Program at MIT and publisher of “Undarkmagazine” (undark.org). In 1992, she won a Pulitzer Prize for a series on primate research, which she turned into a book, “The Monkey Wars.” Her other books include “The Poisoner’s Handbook,” “Ghost Hunters,” “Love at Goon Park,” and “Sex on the Brain.” She has written for publications including the New York Times, Wired, Time, Discover, Mother Jones, the Guardian, and the Boston Globe. Blum is a past president of the National Association of Science Writers, a fellow of the American Association for the Advancement of Science, and a lifetime associate of the National Academy of Sciences.
(To sign up for a free subscription to Food Safety News, click here.)
]]>This pattern of behavior was discovered during an internal audit at the manufacturing location, according to information in the FDA’s Weekly Enforcement Report released yesterday.
“During an internal audit at the manufacturing location, it was discovered that finished product had been released on numerous occasions after receiving presumptive positive test results for Listeria monocytogenes and Salmonella; confirmation was not conducted,” according to the FDA report.
Testing for pathogens such as Listeria monocytogenes and Salmonella involves multiple stages. The “presumptive positive” first stage indicates the possibility that a food sample may contain the pathogen. A second, confirming stage to identify the microbe is standard laboratory operating procedure.
McCain’s Colton manufacturing facility was most recently inspected by the Food and Drug Administration in January, according to information in the agency’s on-line inspection database. That inspection resulted in a classification of “Voluntary Action Indicated,” suggesting that FDA investigators recorded one or more “Inspectional Observations” requiring correction. Food Safety News has requested, but has not yet received, a copy of the Establishment Inspection Report.
On Oct. 13 McCain recalled all products it shipped on or after Jan. 1, 2016, from its Colton, CA, facility. The recalled products were distributed in the United States, Canada, China, Korea and Mexico. The recalls were classified as Class II under FDA’s three-class system. Products that might cause a temporary health problem, or pose only a slight threat of a serious nature are considered to be Class II.
McCain Foods is a privately owned, multinational company with its main offices in Ontario, Canada. It has many business customers, including other food companies, retailers, restaurants and institutional foodservice operations such as school districts. Recalls by retailers that used McCain ingredients in their branded products include WalMart, Trader Joe’s, Whole Foods and 7-Eleven.
To date, no confirmed illnesses have been reported in connection with the recalled products. However, it can take up to 70 days after exposure for symptoms of Listeria infection to develop.
McCain’s voluntary recall included the following 63 different products, packaged and sold under multiple brand names, which are indicated in parentheses.
- Fire Roasted 1/2″ Diced Red Peppers; Sold in 2 lb. and 25 lb. containers (Jon-Lin)
- Fire-Roasted 1/4″ Diced Red Jalapenos; Sold in 25 lb. containers (Jon-Lin and NatureSmart)
- Sautéed 3/8″ Diced Onions; Sold in 25 lb. containers (Jon-Lin and NatureSmart)
- Corn and Black Bean Blend: Sold in 2 lb. and 25 lb. packages (Jon-Lin, NatureSmart and Chef Sensations)
- Caramelized Onion Strips: Sold in 2 lb. and 25 lb. packages (Jon-Lin and NatureSmart)
- Fire-Roasted Red Pepper Julienne Strips: Sold in 2 lb. and 25 lb. packages (Jon-Lin and NatureSmart)
- Fire-Roasted Thin Sliced Portobello Mushrooms: Sold in 2 lb. packages (Jon-Lin and NatureSmart)
- Caramelized 3/8″ Diced Onions: Sold in 25 lb. packages (Jon-Lin)
- Roasted Vegetable Blend: Sold in 2 lb. packages (GFGB)
- Fire Roasted Half Moon Green Zucchini : Sold in 25 lb. and 1,200 lb. packaging (Jon-Lin)
- Fire Roasted Super Sweet Corn: Sold in 20 lb. (Chef Sensations)
- Fire Roasted Red Pepper Strips: Sold in 20 lb. (Jon-Lin)
- Smokehouse Roasted 5/8″ Diced Apples: Sold in 25 lb. (Jon-Lin)
- Fire-Roasted 1/2″ Diced Onions: Sold in 25 lb. (Jon-Lin)
- Fire Roasted 1/2″ Diced Green Peppers: Sold in 25 lb. (Jon-Lin)
- Fire Roasted 1/2″ Diced Yellow Peppers: Sold in 25 lb. (Jon-Lin)
- Fire-Roasted Onion/Pepper Strip Blend: Sold in 20 lb. (Jon-Lin)
- Fire Roasted 1/4″ Diced Red Peppers: Sold in 25 lb. (Jon-Lin)
- Sautéed 1/4” Diced Onions: Sold in 25 lb. (Jon-Lin)
- Fire-Roasted Rubio’s Vegetable Blend: Sold in 2 lb. (Chef Sensations)
- Fire-Roasted Onion Strips: Sold in 20 lb. (Jon-Lin)
- Sautéed Mushrooms: Sold in 500g (no brand name)
- Fire-Roasted Bell Peppers and Onions Sold in 14 oz. package. (Trader Joe’s and unbranded)
- Sautéed Organic Onion Strips: Sold in 25 lb containers (Jon-Lin and NatureSmart)
- Jalapeno Poblano Onion Blend; Sold in 2 lb. and 25 lb. containers. (Jon-Lin)
- Dark Caramelized 3/8″ Diced Sweet Onions; Sold in 20 lb. containers. (Jon-Lin)
- Caramelized 1/4″ Diced Onions; Sold in 25 lb. containers (Jon-Lin)
- Fire-Roasted Homestyle Julienne Tri-Colored Pepper Strips; Sold in 20 lb. containers. (Jon-Lin and NatureSmart)
- Fire Roasted 1/2″ Diced Poblano Chiles: Sold in 25 lb. containers (Jon-Lin)
- Fire-Roasted 1/2″ Diced Onions: Sold in 25 lb. containers (Jon-Lin)
- Sautéed 3/8” Diced Organic Onions: Sold in 25 lb. containers (Jon-Lin and NatureSmart)
- Fire Roasted 1/2″ Diced Green Zucchini ; Sold in 25 lb. containers (Jon-Lin)
- Fire-Roasted Cauliflower Florets ; Sold in 25 lb. containers (Jon-Lin)
- Fire Roasted 1/2″ Diced Tomatoes; Sold in 25 lb. containers. (Jon-Lin)
- Fire Roasted 1/2″ Diced Onion/Pepper Blend; Sold in 25 lb. containers (Jon-Lin)
- Sautéed Julienne Onion Strips With Canola Caramel; Sold in 20 lb. containers (Jon-Lin)
- Pizza Kitchen Cut Medley; Sold in 25 lb. containers (Jon-Lin and NatureSmart)
- Fire-Roasted Pepper and Onion Blend; Sold in 300 lb. containers (Jon-Lin)
- Fire-Roasted 3/8″ Diced Organic Onions; Sold in 25 lb. containers (Jon-Lin and NatureSmart)
- Fire Roasted 1/4″ Diced Poblanos; Sold in 25 lb. containers (Jon-Lin and NatureSmart)
- Fire-Roasted Poblano Strips; Sold in 20 lb. containers. (Jon-Lin and NatureSmart)
- Fire Roasted Red Onion Sticks; Sold in 25 lb. containers (Jon-Lin)
- Caramelized Mushroom Slices; Sold in 25 lb. containers (Jon-Lin)
- Fire-Roasted Rubio’s Elote Corn Blend; Sold in 2 lb. containers (Chef Sensations)
- Fire-Roasted Pineapple Tidbits; Sold in 25 lb. containers (Jon-Lin)
- Fire-Roasted 1/2″ Diced Tomatillos; Sold in 25 lb. containers (Jon-Lin)
- Fire-Roasted Rubio’s Fresno Chili ; Sold in 2 lb. containers (Chef Sensation)
- Fire Roasted Green Pepper Strips ; Sold in 2 lb. containers (Jon-Lin and NatureSmart)
- Fire-Roasted Green Jalapeno Slices; Sold in 20 lb. containers (Jon-Lin and NatureSmart)
- Fire Roasted Yellow Pepper Strips; Sold in 20 lb. containers (Jon-Lin)
- Fire-Roasted Corn & Black Bean Blend; Sold in 2 lb. containers (Jon-Lin and NatureSmart)
- Fire-Roasted Red Onion & Pepper Blend: Sold in 2 lb. containers (Hot Stuff Foods)
- Fire Roasted 1/2″ Diced Button Mushrooms: Sold in 25 lb. containers (Jon-Lin)
- Sautéed Green Pepper Strips: Sold in 25 lb. containers (Jon-Lin)
- Fire-Roasted Home-Style Red Pepper Strips: Sold in 20 lb. containers (Jon-Lin)
- Fire-Roasted Home-Style Green Pepper Strips: Sold in 20 lb. containers (Jon-Lin)
- Fire-Roasted Home-Style Yellow Pepper Strips: Sold in 20 lb. containers (Jon-Lin)
- Fire-Roasted 1/4″ Diced Jalapenos: Sold in 25 lb. containers (Jon-Lin and NatureSmart)
- Fire Roasted 1/2″ Diced Eggplant: Sold in 25 lb. containers (Jon-Lin)
- Fire-Roasted Red Jalapeno Slices: Sold in 2 lb. containers (Jon-Lin)
- Fire-Roasted Red Jalapeno Slices: Sold in 2 lb. containers (Jon-Lin)
- Fire-Roasted 1/2″ Diced Red Onions: Sold in 25 lb. containers (Jon-Lin)
- Fire-Roasted Organic Super Sweet Corn: Sold in 1,500 lb. containers (Jon-Lin)
For additional information on the recalls and investigation, please see:
- McCain Foods confirmed as corn supplier behind multiple recalls in U.S.
- McCain Foods USA expands recall to all products it makes at Colton, CA facility
- Prime Deli Corp. recalls 7-Eleven salads for risk of Listeria, Salmonella in corn
- Trader Joe’s salads, Mary’s Harvest wraps recalled for risk from corn
- Producer recalls Marketside brand salads for risk of contaminated corn
- Salad recall includes Whole Foods 365 brand; corn poses a risk of infection
- Potentially contaminated corn prompts grocery chain to recall the chicken salad
- McCain Foods recall causes Hy-Vee to recall meat, poultry, potato products
(To sign up for a free subscription to Food Safety News, click here.)
]]>The recalled products were manufactured from February 27, 2018, through July 20, 2018, and were sold under the brand names G & C Raw Dog Food and G & C Raw Cat Food.
The expanded recall was initiated after the Ohio Department of Agriculture found Listeria monocytogenes in some G & C finished products.
Recalled products are labeled with lot numbers ending in 022718 through 072018 and include the following:
- Beef Veggie Mix Dog Food
- Ground Beef Dog Food
- Sliced Beef Heart Dog Food
- Ground Beef Heart Dog Food P
- Kim’s Special Beef Organ Dog Food
- Ground Chicken Dog Food
- Chicken Veggie Mix Dog Food
- Chicken Mix Patties Dog Food
- Duck Veggie Mix Dog Food
- Ground Duck Dog Food
- Ground Rabbit Dog Food
- Rabbit Veggie Mix Dog Food
- Ground Lamb Dog Food
- Lamb Veggie Mix Dog Food
- Ground Beef Pancreas Dog Food
- Beef Liver Chunks Dog Food
- Beef Sweet Breads Dog Food
- Ground Pork Dog Food
- Pork Veggie Mix Dog Food
- Shelby’s Pork Organ Mix Dog Food
- Ground Pollock Dog Food
- Turkey Veggie Mix Dog Food
- Ground Turkey Dog Food
- Tripe Dog Food
- Pat’s Cat Beef
- Pat’s Cat Chicken
- Pat’s Cat Turkey
- Pat’s Cat Duck
- Pat’s Cat Rabbit
The products were distributed by direct delivery in Ohio, Michigan, Indiana, Pennsylvania, Kentucky, North Carolina, Tennessee, South Carolina, Georgia, and Illinois.
Listeria monocytogenes can infect pets, although such infections are uncommon. Infected animals may display symptoms including mild to severe diarrhea, anorexia, fever, nervous,
muscular and respiratory signs, abortion, depression, shock, and death. Infected animals may shed Listeria monocytogenes in feces, contaminating the home environment and spreading the infection to humans and to other household pets.
No confirmed illnesses have been associated to date with these recalled products.
G & C is urging consumers who purchased any of these products to return them to G & C Raw, 225 N. West Street, Versailles, OH, for a full refund.
(To sign up for a free subscription to Food Safety News, click here.)
The recalled products, which were shipped to distributors in the US and Canada between May 10, 2017, and August 9, 2018, include:
- Rad Cat Raw Diet Grass-Fed Beef Recipe (1oz sample, 8oz, 16oz, 24oz)
- Rad Cat Raw Diet Free-Range Chicken Recipe (1oz sample, 8oz, 16oz, 24oz)
- Rad Cat Raw Diet Pasture-Raised Lamb Recipe (1oz sample, 8oz, 16oz, 24oz)
- Rad Cat Raw Diet Natural Pork Recipe (1oz sample, 8oz, 16oz, 24oz)
- Rad Cat Raw Diet Free-Range Turkey Recipe (1oz sample, 8oz, 16oz, 24oz)
- Rad Cat Raw Diet Pasture-Raised Venison Recipe (1oz sample, 8oz, 16oz, 24oz)
The expanded recall encompasses Lot numbers 62763 through 63101, inclusive. Affected Best By dates are 10/19/18 through 12/3/19, inclusive. Lot numbers and Best By dates are stamped in ink on the bottom of each container.
Listeria monocytogenes is pathogenic to both humans and animals. Healthy individuals may experience one or more of the following symptoms: nausea, vomiting, diarrhea, abdominal cramping, and fever. The elderly, young children, pregnant women, and individuals with weakened immune systems are at risk of severe complications, including premature births, stillbirths, and death.
Animals infected with the pathogen can exhibit a range of symptoms, including vomiting, diarrhea, fever, muscular or respiratory signs, and anorexia (loss of appetite).
Infected animals can shed Listeria monocytogenes through their feces into the home environment, serving as a source of infection for humans and other animals.
No pet or human illnesses associated with the recalled products have been reported to date.
Radagast has identified a source of potential contamination in its facility and has taken proactive measures to eliminate the source. The company is cooperating fully and sharing information with FDA, according to a spokesperson for Radagast.
Consumers with questions about the recall should contact Radagast Pet Food, Inc. at 1-877-567-3001 Monday – Friday 9:00 am – 5:00 pm Pacific Time, or contact the company through its website at www.RadFood.com
(To sign up for a free subscription to Food Safety News, click here.)
]]>
Prior to issuing a public notice on July 12, the FDA had received sporadic reports involving 30 dogs and 7 cats over a three-year period. During that same period, the veterinary cardiology community received about 150 similar reports. In dogs, the disease results in an enlarged heart.
Some of the dogs exhibited signs of heart disease, including decreased energy, cough, difficulty breathing, and episodes of collapse. Reports received by FDA identified a range of brands and formulas.
The common element in these foods appears to be the presence of legumes (including peas, beans, lentils, chickpeas, soybeans, peanuts), pulses (seeds of legumes), and/or potatoes as main ingredients in the pet foods. The list also encompasses protein, starch or fiber derived from legumes.
Since the July 12 notification, FDA has received additional reports, which it is in the process of evaluating. None of these reports involve cats. According to a spokesperson from FDA, the agency is not able to provide an accurate accounting at this time, as the number of reports is continuing to rise and the information is being analyzed as it is received.
Some dog breeds are genetically susceptible to developing DCM. However, at least some of the initial reports to FDA involved other breeds of dog not typically prone to this disease. FDA is evaluating various possible dietary causes of DCM in dogs, including, nutritional makeup of the main ingredients or how dogs process them, main ingredient sourcing, processing, and amount used.
At this point in its investigation, FDA is not advising dog owners to make any dietary changes. Pet owners whose dogs are showing any symptoms of DCM or other heart conditions should contact their veterinarian.
FDA is encouraging pet owners and veterinary professionals to report cases of DCM in dogs suspected of having a link to diet by using the electronic Safety Reporting Portal or calling their state’s FDA Consumer Complaint Coordinators.
(To sign up for a free subscription to Food Safety News, click here.)
]]>The recalled products are:
- Pat’s Cat Turkey Cat Food packed in 1-pound clear plastic containers; Lot #WWPKTF051618
- Ground Lamb Dog Food packed in 2-pound plastic containers; Lot #MFF022718
G&C is based in Versailles, OH. The company distributed the products in Ohio, Michigan, Indiana, Pennsylvania, Kentucky, North Carolina and Georgia through direct delivery.
Listeria monocytogenes can cause illness in susceptible dogs and cats, producing symptoms such as diarrhea, anorexia, fever, nervous and respiratory issues, spontaneous abortion of pregnant animals, depression, shock, and even death. Infected animals can shed the bacteria through their feces, potentially contaminating the home environment and transmitting the infection to their human caregivers.
In humans, Listeria monocytogenes is capable of producing a range of symptoms, depending on the susceptibility of the individual. Healthy people may experience mild symptoms, which can include nausea, vomiting, aches, fever or diarrhea. Pregnant women, young children, the elderly, and individuals with compromised immune symptoms are susceptible to serious and sometimes fatal complications, including miscarriage, premature birth, and death. Babies born to mothers who were infected with Listeria monocytogenes during pregnancy can be born infected.
No human or animal illnesses associated with the recalled products have been reported to date. Consumers who have been in contact with the recalled products and are experiencing any symptoms of Listeria monocytogenes infection should contact a healthcare provider. It can take up to 70 days after exposure to the bacteria for symptoms to develop in humans.
Pets who have consumed either of the recalled products and who are exhibiting symptoms of illness should be seen by a veterinarian.
The company has ceased the production and distribution of the product as its owners investigate what caused the problem.
Consumers who have purchased Pat’s Cat Turkey Cat Food with the lot number, WWPKTF051618, OR Ground Lamb Dog Food with the lot number MFF022718 are urged to return it to G&C Raw, 225 N. West Street, Versailles, OH, for a full refund. Consumers with questions may contact: G&C Raw LLC at 937-827-0010, or by email at [email protected].
(To sign up for a free subscription to Food Safety News, click here.)
]]> Even though the owners of Evanger’s Dog and Cat Food Co. Inc. had a state license to process horse meat from Feb. 14, 2016, through Aug. 17, 2017, they denied any knowledge of how horse meat found its way into their products.
Even though the owners of Evanger’s Dog and Cat Food Co. Inc. had a state license to process horse meat from Feb. 14, 2016, through Aug. 17, 2017, they denied any knowledge of how horse meat found its way into their products.
The pet food company’s license application submitted to the Illinois Department of Agriculture described Evanger’s operations as “canning of pet food” and indicated the horse meat would be sold as “canned pet food” according to information obtained from the department in response to a Freedom of Information Act request.

It is not illegal to use horse meat as pet food in the United States, as long as it’s presence is disclosed on the labeling, a spokesperson for the Food and Drug Administration said.
Evanger’s horse meat processing activities predated the issuance of the state license.
In April 2015, the Illinois agriculture department received a complaint from the Wheeling, IL, fire marshal about improper food storage and disposal, clogged and maggot-filled grease traps, and other unsanitary conditions at Evanger’s Wheeling Road facility. The state initiated an inspection of the facility on May 27, 2015.
During the investigation of the company’s operations, inspectors noted the manufacture of a dog food with horse meat as an ingredient.
As a result of conditions observed during the May inspection, the state of Illinois filed a formal complaint against Evanger’s, charging the company with violations of the Illinois Commercial Feed Act of 1961, the Illinois Food, Drug, and Cosmetic Act, and the Illinois Horse Meat Act.
The complaint stated that Evanger’s “… canned, packed or otherwise processed or prepared for sale a pet food with horsemeat listed as its main ingredient” without having secured a license to do so, and that the company did so under unsanitary conditions.
According to information contained in the report on a follow-up inspection, conducted in January 2016, Evanger manufactured only canned dog and cat foods in the Wheeling facility, receiving raw and frozen pork, chicken, beef and horse meats. The horse meat was imported from foreign countries, the report stated.
One year later, in January 2017, FDA launched an investigation into pentobarbital contamination in a canned, wet dog food manufactured by Evanger’s in June 2016. The investigation was in response to a consumer complaint following the death of a dog who had been fed some “Evanger’s Hunk of Beef au Jus” dog food.
During the investigation, FDA arranged for lab tests to determine the species of meats present in several samples of the same batch of food eaten by the dead dog. Traces of horse antigens were found in one of the samples.
As reported by Food Safety News in February 2017, those level of horse antigens would be consistent with incidental cross-contamination if horse meat was processed in the same facility as the beef-based dog food.
The owners of Evanger’s denied any knowledge of how horse meat had found its way into their products, and cast blame on their beef supplier, Bailey Farms LLC. On April 25, 2017, Evanger’s filed a lawsuit against Bailey, alleging that it had shipped pentobarbital-contaminated horsement instead of the “pet food quality beef” ordered by Evanger’s.
At the time the lawsuit was filed, and throughout the entire period during which the company manufactured dog food that was later found to be adulterated with pentobarbital, Evanger’s license to process horse meat was active and in force.
Evanger’s horse meat license expired in August 2017 and has not been renewed.
(To sign up for a free subscription to Food Safety News, click here.)
]]>